Olushola S. Ayanda 1, Folahan A. Adekola 2
1Department of Chemistry, Faculty of Applied Sciences, Cape Peninsula University of Technology, P.O Box 1906, Bellville, South Africa
2Department of Chemistry, University of Ilorin, P.M.B 1515, Ilorin, Nigeria
Correspondence to: Olushola S. Ayanda , Department of Chemistry, Faculty of Applied Sciences, Cape Peninsula University of Technology, P.O Box 1906, Bellville, South Africa.
| Email: |  |
Copyright © 2012 Scientific & Academic Publishing. All Rights Reserved.
Abstract
This paper presents the kinetics of dissolution of a Nigerian columbite mineral ore sample in hydrochloric acid media. The effects of acid concentration, process temperature, stirring rate and particle size on the dissolution rate were examined. Experimental results showed that the columbite dissolution increases with increasing acid concentration, temperature, stirring speed and decreases with particle size. With 4.0 M HCl solution, about 15.37% of columbite was dissolved within 120 minutes using < 0.15 mm particle size at a temperature of 80℃ and a stirring speed of 360 rpm. The result of the study indicated that the leaching data fitted a diffusion model. Values of 0.5, 22.40 kJmol-1 and 1.51 X 10-5 min-1 were calculated as reaction order, activation energy and Arrhenius constant, respectively for the dissolution process.
Keywords:
Columbite, Kinetics, Niobium, Hydrochloric Acid, Activation Energy , Arrhenius Constant
1. Introduction
Columbite is a black mineral group that is an ore of niobium and tantalum[1, 2]. It contains a higher percentage of niobium as compared to tantalum and as a result it is taken exclusively as niobium mineral. The mineral Niobium is therefore used mainly as an alloy of steels, heat sensitive detective devices called barometer for jet engines and other aircraft components. It is used in electronic devices like cell phones and DVD players. High purity niobium is utilized to fabricate radio frequency cavities for the acceleration of electrons in quantum physics. It is also employed in the field of applied superconductivity, mainly includingenergy-related applications, transportation, computersandinstrumentation. Niobium alloys such as NbTi alloys and Nb3Sn intermetallics, have been the superconducting materials commercially available[3, 4].The processing route for tantalum/niobium ores hasbeen the dissolution of the mineral in hot, concentrated hydrofluoric acid, the metals are then separated either by fractional crystallisation as K2MF7 salts or by solvent extraction[5]. Authors have therefore investigated the effect of mechanical activation on the leaching of columbite and have found that the mechanical activation of niobium /tantalum concentrates markedly affects the rate and extent of the dissolution inhydrofluoric acid media[6] and in a sodium fluoride and hydrofluoric acid solution[5].Rodriguez et al.[7] also investigated the leaching of columbite in hydrofluoric and carboxylic acids media. It is therefore interesting to know the leaching mechanism of Nb and Ta bearing minerals i.ecolumbite in acid solutions that are not as strong as HF. Ayanda et al.[1] therefore reported the dissolution kinetics of a Nigerian columbite mineral ore sample in nitric acid and values of 15.100 kJ mol-1 and 9.42 × 10-5 min-1 were obtained for the activation energy and Arrhenius constant of the columbite dissolution in nitric acid, respectively and the order of reaction was 0.35.The present investigation was therefore aimed atestablishing the conditions for the leaching of the same columbitemineral ore in hydrochloric acid media.
2. Experimental
2.1. Material
Columbite sample used for this study was collected from a mining site inDaba-Lema, Edu Local Government of Kwara State. All reagents used were of analar grade and were all products of BDH. De-ionized water was used for all the analytical preparations.
2.2. Leaching Procedure
0.5g of columbite sample was leached in100 ml of hydrochloric acid. The fraction of columbite sampledissolved for each process was calculated by the expression[8, 9]: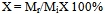 | (1) |
where Mr = Mi – Mf, Mi – initial mass of columbite before leaching, Mf – final mass of columbite after leaching, Mr – mass of columbite dissolved.
2.3. Kinetic Analysis
For this study, three shrinking core models[1, 10, 11] were investigated on the result obtained on the effect of temperature. 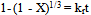 | (2) |
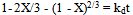 | (3) |
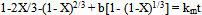 | (4) |
where X is the fraction of columbite dissolved; t is contact time (s); kr, kd and km are the reaction rate constants. Equation 3 is based on the chemically controlled reaction and its slope corresponds to the apparent rate constant kr. Equation 4 on the other hand is based on diffusion controlled and its slope gives the apparent rate constant kd. Lastly, Equation 3 is based on a mixed controlled reaction and apparent rate constant km.The slope that gave the best correlation coefficient was chosen as the model that fit perfectly with the columbite dissolution
3. Results and Discussion
3.1. Leaching Studies
3.1.1.Effect of Concentration
The effect of concentration on columbitesampledissolution was studied over the concentration range of 0.1M–8.0M. The results obtained are as shown in Figures 1. It was observed from Figure 1 that an increase in the concentration of hydrochloric acid was accompanied with increase in the rate of columbite dissolution. i.e the rate of columbite dissolution is affected directly by hydrogen ion[H+] concentration. This agrees with several reported work on acid dissolution of solid mineral ores[12-15]. The result also showed that 11.04% columbite was dissolved by contacting columbite sample with 8.0M HCl at a temperature of 55℃ and a contact time of 120 min.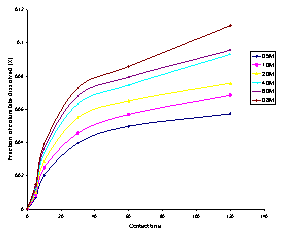 | Figure 1. Fraction of columbite dissolved (X) versus contact time (min) at different concentrations of HCl. Experimental conditions: Temperature = 55℃, mass of columbite used = 0.5g, stirring rate = 200rpm, particle size = <0.15mm |
3.1.2.Effect of Temperature
The effect of temperature was studied over thetemperature ranges of 28 – 80℃ and investigated in different concentrations of leachants ranging from 0.5M-8.0M. The results obtained are presented in Figure 2.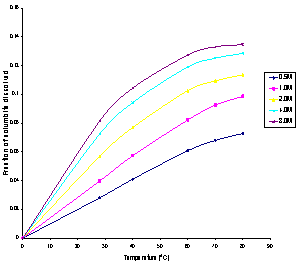 | Figure 2. Fraction of columbite dissolved (X) versus temperature (℃) at different concentration of HCl. Experimental conditions: Mass of columbite used = 0.5g, elapse time: 120 min, stirring rate = 200rpm, particle size = <0.15mm |
The figure showed that increase in temperature accelerates the reaction rate and this leads to increase in the amount of columbite dissolved. 13.46% of columbite sample was dissolved in 8.0M HCl at a temperature of 80℃ and a contact time of 120min.
3.1.3.Effect of Particle Size
The result of the effect of particle size on the dissolution of columbite sample as investigated in 4.0M HCl is represented in Figure 3.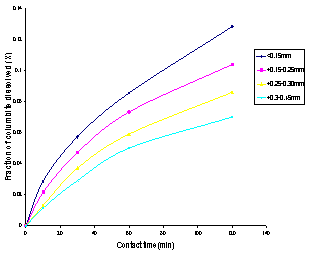 | Figure 3. Fraction of columbite dissolved versus contact time for different particle size fraction of columbite |
A graph of the fraction of columbite dissolved versus contact time for the different particle size fraction as represented in Figure 3, showed that the fraction with the smallest particle size (<0.15mm) gave the highest percentage of columbite dissolution (12.86%). This is due to the highest surface area of the smallest particle size fraction.
3.1.4.Effect of Stirring Speed
The percentage of columbite dissolved (%) versus stirring rate (rpm) as investigated between 0 – 540 rpm is as shown in Figure 4.The result showed that the amount of columbite dissolved increases with stirring speed between 0 – 360rpm. The percentage dissolved appears to be practically constant afterwards. About 15.37% of columbite was dissolved in 4.0 M HCl, at a temperature of 80℃, and a stirring speed of 360 rpm.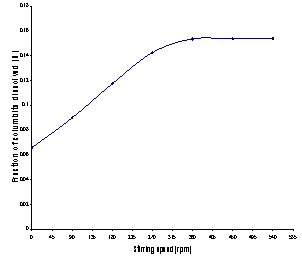 | Figure 4. Fraction of columbite dissolved (%) versus stirring rate (rpm) |
| Table 1. The kr, kd and km and correlation coefficients value at various temperatures of HCl forcolumbite sample dissolution |
| | Temperature (℃) | Apparent rate constant (min-1)kr1kd1km1 | Correlation coefficient (R2)kr1kd1km1 | | 28 | 1.33X10-43.48X10-6 1.38X10-4 | 0.9050 0.9993 0.9117 | | 40 | 1.77X10-4 6.09X10-6 1.85X10-4 | 0.9259 0.9981 0.9369 | | 60 | 1.72X10-4 8.70X10-6 1.82X10-4 | 0.9626 0.9951 0.9644 | | 70 | 1.73X10-4 1.22X10-5 1.84X10-4 | 0.9934 0.9946 0.9931 | | 80 | 1.65X10-4 1.30X10-5 1.77X10-4 | 0.9917 0.9925 0.9938 |
|
|
3.2. Kinetics of Dissolution
The rate constant values kr, kd and km calculated from Equation (2), (3) and (4) respectively, and theircorrespondng correlation coefficients are given in Table 1. The values of the correlation coefficients indicated that the dissolution rate of columbite in hydrochloric acid is diffusion controlled. The application of the diffusion kinetic model is as shown in Figure 5.
3.2.1. Activation energy
This model was chosen for further studies. Table 2 shows the values for the apparent rate constants and ln of the apparent rate constants for HCl at various temperatures and the Arrhenius plot is illustrated in Figure 6.From the slope of Figure 6, using Equation 5, the activation energy (Ea) for the dissolution of columbite in HCl is 22.40 kJmol-1.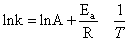 | (5) |
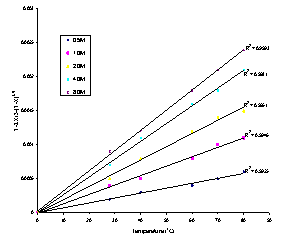 | Figure 5. Plot of 1-2/3X-(1-X)2/3 versus contact time at various temperatures of HCl |
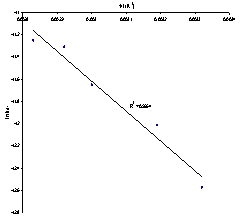 | Figure 6. Plot of Plot of ln kd1 versus 1/T(K-1) |
| Table 2. Rate constants for HClat various temperatures |
| | T℃ 1/T (K-1) | Hydrochloric Acid | | kd1 | lnkd1 | | 28 | 3.32 X10-3 | 3.48X10-6 | -12.57 | | 40 | 3.19 X10-3 | 6.09X10-6 | -12.01 | | 60 | 3.00 X 10-3 | 8.70X10-6 | -11.65 | | 70 | 2.92 X 10-3 | 1.22X10-5 | -11.31 | | 80 | 2.83 X 10-3 | 1.30X10-5 | -11.25 |
|
|
3.2.2. Arrhenius Constant
The extrapolation of Figure 6 to the y-axis[Figure 7] gave a value of -11.1 min-1 which is equivalent to lnA in Equation 5. The Arrhenius constant (A) for the columbite dissolution in HCl is 1.51 X 10-5 min-1.
3.2.3. Order of reaction
The order of reaction from the slope for columbite dissolution in hydrochloric acid with respect to[H+] was 0.5.Table 3 shows the values for the apparent rate constants for the hydrogen ion concentration while Figure 8 shows the plot of lnkd2 versus ln[H+] for hydrochloric acid concentrations.| Table 3. Rate constants for HCl at various concentrations |
| | [H+]ln[H+] | Hydrochloric Acid | | kd2 | lnkd2 | | 0.5 | -6.93 X10-1 | 3.19 X10-6 | -12.66 | | 1.0 | 0 | 4.60 X10-6 | -12.29 | | 2.0 | 6.73 X 10-1 | 5.64 X10-6 | -12.09 | | 4.0 | 1.39 | 8.55 X10-6 | -11.67 | | 6.0 | 1.79 | 9.40 X10-6 | -11.57 | | 8.0 | 2.08 | 1.20 X10-5 | -11.33 |
|
|
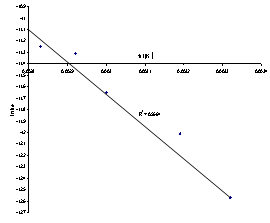 | Figure 7. Extrapolation of the plot of ln kd1 versus 1/T(K-1) |
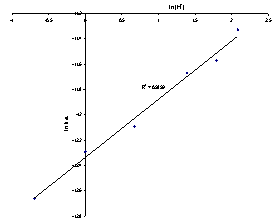 | Figure 8. Plot of ln kd2 versus ln[H+] |
4. Conclusions
In the present study, the dissolution of columbite in hydrochloric acid was studied. It was found that the rate of columbite dissolution increases with acid concentration, temperature, stirring speed and decreases with particle size. The dissolution of columbitein hydrochloric acid was found to be controlled by the shrinking core model for a diffusion-controlled process. Values of 22.40kJmol-1 and 1.51 X 10-5 min-1 were obtained for the activation energy and Arrhenius constant of columbite dissolution in hydrochloric acid respectively; and the order of reaction was 0.5.
References
| [1] | Olushola S Ayanda, Folahan A Adekola and Olalekan S Fatoki, “Dissolution Kinetics of Columbite in Nitric Acid”, Asian Journal of Chemistry, Vol. 24, No. 3, 1087-1090, 2012. |
| [2] | Olushola S Ayanda, Folahan A Adekola, "Comparison of Some Physicochemical Characterization of Columbite and Tantalite Samples from Different Locations in Nigeria". Chemistry for Sustainable Development, Vol. 19, pp. 243-247, 2011. |
| [3] | Htwe H Htet and Kay T Lwin, “Study on Extraction of Niobium Oxide from Columbite–Tantalite Concentrate”, World Academy of Science, Engineering and Technology, Vol. 46, pp. 133-135, 2008. |
| [4] | Olushola S Ayanda, Folahan A Adekola, "A Review of Niobium-Tantalum Separation in Hydrometallurgy". Journals of Minerals and Material Characterization and Engineering”, Vol. 10, No. 3, pp. 245-256, 2011. |
| [5] | NJ Welham, “Enhanced dissolution of tantalite/columbite following milling”, International Journal of Mineral Processing, Vol. 61, Issue 3, pp. 145–154, 2001. |
| [6] | H Hoberg, J Götte, “The influence of mechanical activation on the kinetics of the leaching process of columbite” Int. J. Miner. Process., Vol. 15, pp. 57–64, 1985. |
| [7] | M Rodriguez, J Rivarola, Maria C Ruiz, “The effects of carboxylic acid addition on hydrofluoric acid autoclave leaching of a ferrocolumbite”, Hydrometallurgy, Vol. 74 Issues 1-2, pp. 39-46, 2004. |
| [8] | Bryn E Kimball, Donald J Rimsstidt, Susan L Brantley, “Chalcopyrite dissolution rate laws” Applied Geochemistry, Vol. 25, Issue 7, pp. 972-983, 2010. |
| [9] | A Baba, F Adekola, D Ayodele, “Study of metals dissolution from a brand of mobile phone waste”, Association of Metallurgical Engineers of Serbia, Vol. 16, No. 4, pp. 269-276, 2010. |
| [10] | Greg W Dicinoski, Lawrence R Gahan, Peter J Lawson, John A Rideout, “Application of the shrinking core model to the kinetics of extraction of gold(I), silver(I) and nickel(II) cyanide complexes by novel anion exchange resins”, Hydrometallurgy, Vol. 56, Issue 3, pp. 323–336, 2000. |
| [11] | Folahan A Adekola, Alafara A Baba, Olushola S Ayanda, “A Study of the Kinetics of Dissolution of a Nigerian Laterite in Sulphuric Acid”, Pacific Journal of Science and Technology, Vol. 11, No. 1, pp. 373-382, 2010. |
| [12] | EO Olanipekun, “A kinetic study of the leaching of a Nigerian ilmenite ore by hydrochloric acid”, Hydrometallurgy, Vol. 53, pp. 1-10, 1999. |
| [13] | EO Olanipekun, “Quantitative leaching of galena”, Bull. Chem. Soc. Ethiopia. Vol. 14, No. 1, pp. 25-32, 2000. |
| [14] | MM Antonijevi, ZD Jankovi, MDDimitrijivi, “Kinetics of chaloopyrite dissolution by hydrogen peroxide in sulphuric acid”, Hydrometallurgy, Vol. 71, Issues 3-4, pp. 329-334, 2004. |
| [15] | G Gulfen, M Gulfen, Ali O Aydiin, “Dissolution Kinetics of Iron from diasporic Bauxite in Hydrochloric acid solution”, Indian Journal of Chemical Technology, Vol. 13, pp. 386-390, 2006. |
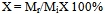
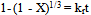
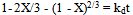
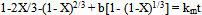


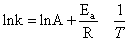




 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-Text HTML
Full-Text HTML
