-
Paper Information
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
International Journal of Metallurgical Engineering
2012; 1(2): 28-34
doi: 10.5923/j.ijmee.20120102.04
Solvent Extraction and Separation of Copper and Zinc from a Pickling Solution
Manish K. Sinha , S. K. Sahu , Pratima Meshram , B. D. Pandey , V. Kumar
Metal Extraction & Forming Division, CSIR-National Metallurgical Laboratory, Jamshedpur , 831 007, India
Correspondence to: S. K. Sahu , Metal Extraction & Forming Division, CSIR-National Metallurgical Laboratory, Jamshedpur , 831 007, India.
| Email: |  |
Copyright © 2012 Scientific & Academic Publishing. All Rights Reserved.
Solvent extraction studies of copper and zinc have been carried out using Versatic 10 acid and Cyanex 272 separately from a model brass pickle liquor. Various parameters for the extraction and separation of copper and zinc such as effect of pH, extractant concentration, phase ratio etc. have been optimized. It was observed that copper was almost completely extracted into the organic phase comprising of 30% Versatic 10 acid at the equilibrium pH of 5.0 using the phase ratio of 1:1 whereas, zinc extraction was noticed at above pH 5.0. On the other hand the pH0.5 values were 3.5 and 4.6 for zinc and copper respectively with 20% Cyanex 272. The difference in pH0.5 value of 1.10 indicated the possible separation of Zn and Cu. By McCabe Thiele diagram number of stages required for the counter current extraction of copper and zinc has been determined for both the solvents. The stripping study showed that 1 mol/L H2SO4 was sufficient to strip metal ions in a single contact from each of the extractant.
Keywords: Solvent extraction, Pickling solution, Versatic 10 acid, Cyanex 272, Copper, Zinc
Article Outline
1. Introduction
- Brass tends to build up a black coating on the surface called tarnish which is essentially a type of corrosion that is caused by exposure to the air. Therefore, cleaning of the surface becomes increasingly necessary. The cleaning process involves removal of rust and oxide impurities by dipping the material in dilute sulphuric acid solution, which is usually reused several times before its disposal as a waste. As a result the waste pickle liquor is loaded with pollutant materials such as copper, zinc, chromium etc. Due to its environmentally hazardous nature it cannot be disposed off without pretreatment. Recovery of these metals is consistent with the goals of waste management strategy since they could provide some economical benefits and solve the pollution problem too. To obtain valuable end-products like copper powder, copper oxide, zinc oxide and copper/zinc salt from such streams solvent extraction is considered an ideal technique which has been applied extensively.Several workers have carried out numerous investigations for the extraction and separation of different metals such as copper, zinc etc. for the processing of sulphate solutions of various low grade materials or wastes, such as brass ash, converter slag and complex ores using different organic extractants. The separation of copper and zinc from leach solution of a complex sulphide ore was studied by Kumaret al.[1] and Pandey et al.[2]. The results show that LIX 64N selectively extracted copper from a copper- zinc solution. The raffinate containing zinc was purified and the metal was produced by electrowinning. Kumar et al.[3] compared the efficiency of LIX 84 and LIX 64N for the separation of copper and zinc from a solution containing impurities like iron and manganese; LIX 84 showed greater selectivity for copper/zinc separation compared to LIX 64N. Similarly, Reddy and Priya[4,5] developed a process for separation of Cu(II), Ni(II) and Zn(II) using LIX 84I and observed that metal extraction depend upon pH and temperature had no effect on the extraction of metal. Copper and zinc separation from bioleaching solution was carried out by Zhuo-yue et al.[6] using LIX 984 and D2EHPA, and various parameters for extraction were optimized. The separation of divalent metal ions from a synthetic solution containing Zn, Cu, Ni, Co, and Mg with D2EHPA was studied by Cheng [7]. The extraction order was found to be: Zn2+ > Ca2+ > Mn2+ > Cu2+ > Co2+ > Ni2+ > Mg2+ which showed possible separation of copper from zinc. Sole and Hiskey[8] reported the extractive nature of Cyanex 272, Cyanex 302 and Cyanex 301 towards extraction of copper and also studied the nature of extracted species in all the cases. The extraction of copper decreases in the order: Cyanex 272 > Cyanex 302 > Cyanex 301. The trend for metal extraction using Cyanex 272 was found to be Fe < Zn < Cu < Co < Ni. The extraction behavior of Cyanex 301, Cyanex 302 and their binary mixture with Aliquat 336 towards Cu, Zn, Co, Fe, Mn and Ni was studied by Tait [9]. It was found that Cyanex 302/Aliquat 336 can be used as a potential solvent mixture for copper/zinc separation. Konglo et al.[10] studied the cobalt and zinc recovery from copper sulphate solution by selective extraction of copper by LIX 984 followed by extraction of cobalt and zinc with D2EHPA. Separation of copper and zinc from Hudson Bay mining and smelting discharge was studied by Owusu [11] using LIX 622. He observed that upto 97-98% Cu was extracted with negligible co-extraction of Zn, Fe, Cd and Co. A process for the metal extraction using organophosphorous or carboxylic acid as extractant in the acidic range was patented by Preston et al.[12]. The synergistic effect between extractant and non chelating aldehyde oxime has enabled extraction to take place at lower pH. Dukov and Guy [13] investigated the extraction of copper and zinc by using LIX 34 and Versatic 911 acid and their mixtures, and observed that no synergism was found when Versatic acid was added. Zinc and copper can also be extracted from sulphate media by carboxylic acids[14]. The relative order of extraction with increasing pH was found to be: Fe(III) < Cu < Zn < Ni < Co < Mn < Ca < Mg. Copper and zinc recovery from spent copper pickle liquor was studied by Mahmoud et al.[15] using Acorga 5640 for selective extraction of copper followed by recovery of chromium and zinc by precipitation. In the present work separation and extraction of copper and zinc from a model brass pickle liquor were investigated by using Versatic 10 acid and Cyanex 272.
2. Materials and Methods
- A typical brass pickle liquor generated at a copper/brass industry in India contains 45.1 g/L H2SO4, 25 g/L Zn, 35 g/L Cu(II), 1.1 g/L Cr(III), 0.2 g/L Fe(Total), 0.01 g/L nickel and 134.75 g/L total sulphate. A solvent extraction process was developed to separate copper and zinc using Versatic 10 acid and Cyanex 272 as extractants. Synthetic solutions containing 35 g/L Cu or 30 g/L Zn were prepared by dissolving required amount of CuSO4 and ZnSO4 in distilled water and used in the solvent extraction studies. Kerosene was used as the diluent and with Cyanex272, isodecanol was used as the phase modifier. Dilute sulfuric acid was used as stripping agent for the stripping of metal ions from the loaded organic.All solvent extraction experiments were carried out by shaking equal volume (except for the McCabe Thiele construction) of synthetic aqueous solution and desired extractant of known concentration in a separating funnel for 15 min. which was found to be sufficient to reach equilibrium. The pH of the aqueous solution was adjusted to the desired value by adding dilute H2SO4 or NaOH before equilibrium. After the phase disengagement the aqueous and organic phases were separated. Metal ion concentration in the aqueous phase was analyzed by Atomic Absorption Spectrophotometer (AAS). Metal contents of the organic phases were determined by mass balance. Stripping studies of metal ions from the loaded organic phase was carried out with dilute sulfuric acid.In order to apply the solvent extraction and separation process so developed to the real spent brass pickle liquor, a model synthetic solution containing 45 g/L H2SO4, 30 g/L Zn, 35 g/L Cu, 0.5 g/L Cr, 0.2 g/L Fe and 0.03 g/L Ni was prepared. Acid, from the model solution was recovered by solvent extraction with tris(2-ethylhexyl) amine (TEHA) (Results not included in this paper). Iron was then removed by precipitation by blowing air at pH 3.5 and temperature 60 oC for 1h. The resulting solution containing 30 g/L Zn , 35 g/L Cu, 0.5 g/L Cr and 0.03 g/L Ni was used to simulate the separation of copper and zinc by solvent extraction with Versatic 10 acid and Cyanex 272.
3. Results and Discussion
3.1. Solvent Extraction Separation of Copper and Zinc with Versatic 10 Acid
3.1.1. Effect of pH
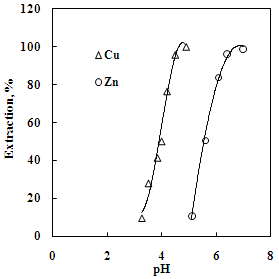 | Figure 1. Effect of pH on the solvent extraction of copper and zinc.Aq. phase: 35 g/L Cu, 30 g/L Zn, Org. phase: 30 % Versatic 10 acid in kerosene |
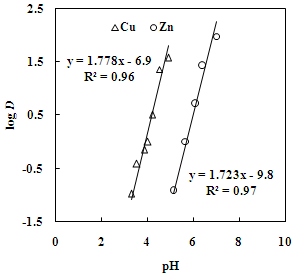 | Figure 2. Effect of pH on the distribution ratio of copper and zinc.Aq. phase: 35 g/L Cu, 30 g/L Zn, Org. phase: 30 % Versatic 10 acid in kerosene |
3.1.2. Effect of Extractant Concentration
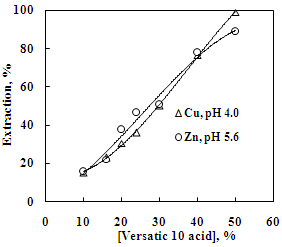 | Figure 3. Effect of Versatic 10 acid concentration on the solvent extraction of copper and zinc. Aq. phase: 35 g/L Cu and 30 g/L Zn, Org. phase: Different conc. of Versatic 10 acid in kerosene |
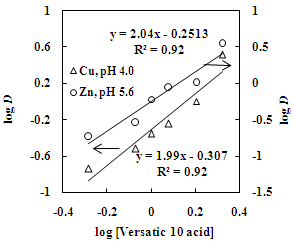 | Figure 4. Effect of Versatic 10 acid concentration on the distribution ratio of copper and zinc. Aq. phase: 35 g/L Cu and 30 g/L Zn, Org. phase: Different conc. of Versatic 10 acid in kerosene |
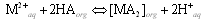 | (1) |
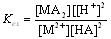 | (2) |
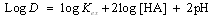 | (3) |
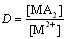 | (4) |
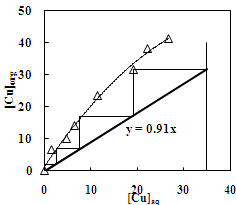 | Figure 5. McCabe –Thiele plot for the extraction of copper with Versatic 10 acid. Aq. phase: 35 g/L Cu Org. Phase: 30 % Versatic10 acid in kerosene |
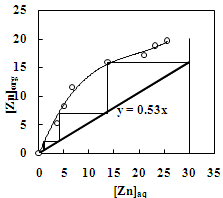 | Figure 6. McCabe –Thiele plot for the extraction of zinc with Versatic 10 acid. Aq. phase: 30 g/L Zn, Org. Phase: 30 % Versatic 10 acid in kerosene |
3.1.3. Effect of Phase Ratio Variation
- In order to determine the extraction capacity of 30% Versatic 10 acid, the extraction of copper and zinc from sulphate solution at different O/A ratio was studied at the equilibrium pH of 4.0 and 5.6 respectively. The percentage extraction of copper increased from 18 to 96% whereas, zinc extraction increased from 13% to a maximum of 87% with the increase in phase ratio from 0.2 to 5. Loading capacity of 30% Versatic 10 acid was found to be 34.95 g/L and 19.60 g/L for Cu and Zn respectively. Number of counter-current stages required for complete metal extraction can be predicted by the use of McCabe Thiele diagram. Extraction isotherm for copper indicates that a total of four stages are required for complete extraction of copper whereas, complete extraction of zinc could be achieved in three stages using the phase ratio of 1.1:1 and 1.9:1 respectively (Figure 5 and Figure 6).
3.2. Solvent Extraction separation of Copper and Zinc with Cyanex 272
- Acidic form of the extractant alkyl phosphate Cyanex 272 often exists in the dimer form[15]. Hence, the metal extraction using Cyanex 272 (HA) can be expressed by the following reactions:
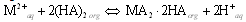 | (5) |
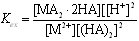 | (6) |
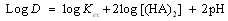 | (7) |
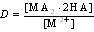 | (8) |
3.2.1. Effect of pH
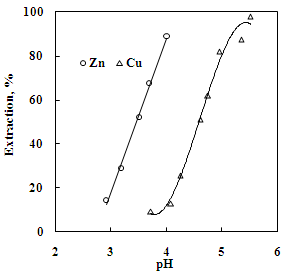 | Figure 7. Effect of pH on the solvent extraction of copper and zinc. Aq. phase: 30 g/L Zn,35 g/L Cu, Org. Phase: 20 % Cyanex 272 in kerosene |
3.2.2. Effect of Extractant Concentration
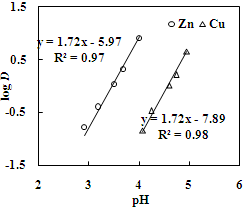 | Figure 8. Effect of pH on the distribution ratio of copper and zinc. Aq. phase: 30 g/L Zn,35 g/L Cu, Org. Phase: 20 % Cyanex 272 in kerosene |
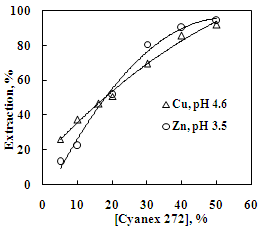 | Figure 9. Effect of Cyanex 272 concentration on the solvent extraction of copper and zinc. Aq. phase: 30 g/L Zn, 35 g/L Cu, Org. Phase: Different concentration of Cyanex 272 in kerosene |
3.2.3. Effect of Phase Ratio Variation
- The effect of O/A ratio on the extraction of zinc and copper under the condition of equilibrium pH of 3.5 and 4.6, respectively using 20% Cyanex 272 (0.63M) showed that the percentage extraction of zinc increased from 20% to 97% whereas the percentage extraction of copper increased from 21% to 98% with the increase in O/A phase ratio from 0.2 to 5. Loading capacity of 20% Cyanex 272 was found to be 34.95 g/L and 29.35 g/L for Cu and Zn, respectively. Number of counter current extraction stages required for complete extraction of zinc and copper from the sulphate solution was determined from McCabe Thiele plot. It was found that 3 counter extraction stages were required for complete recovery of zinc and copper with 20% Cyanex 272 solution at O/A = 1.8:1 phase ratio (Figure 11 and 12).
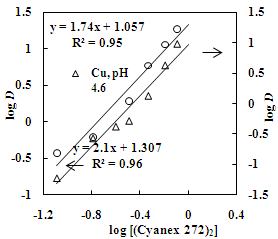 | Figure 10. Effect of Cyanex 272 concentration on the distribution ratio of copper and zinc. Aq. phase: 30 g/L Zn, 35 g/L Cu, Org. Phase: Different concentration of Cyanex 272 in kerosene |
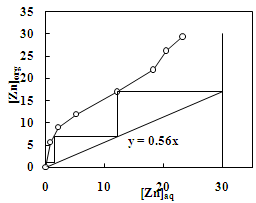 | Figure 11. McCabe-Thiele Plot for the extraction of zinc with Cyanex 272. Aq. phase: 30 g/L Zn, Org. Phase: 20 % Cyanex 272 in kerosene |
3.3. Stripping of Metal Ions from the Loaded Organic Phase
- Stripping studies of copper and zinc from the loaded Versatic 10 acid and Cyanex 272 was carried out by repetitively contacting the organic phases with dilute sulfuric acid solutions separately. The results of stripping studies are given in Table 1. It was observed that 0.25M H2SO4 stripped 50% and 46% of copper and zinc respectively from the loaded Versatic 10 acid whereas complete stripping of copper and zinc was achieved in a single contact using 1.0M sulphuric acid. Complete stripping of copper and zinc from the loaded Cyanex 272 was achieved using 0.5M H2SO4 in a single contact at a phase ratio of 1. The stripped metals can be recovered as salts by evaporation-crystallization or metal cathodes powder by electrowinning[18].
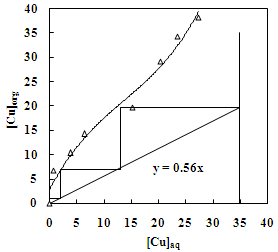 | Figure 12. McCabe -Thiele Plot for the extraction of copper with Cyanex 272. Aq. phase: 35 g/L Cu, Org. Phase: 20 % Cyanex 272 in kerosene |
3.4. Recovery of copper and zinc from acid and iron depleted spent brass pickle liquor
- The optimum conditions obtained for the extraction and separation of copper and zinc from their mixed solution, were applied to the model solution with a chemical composition similar to that of the acid and iron depleted real brass pickle liquor (Zn(II): 30 g/L, Cu(II): 35 g/L, Cr(III): 0.5 g/L, Ni(II): 0.03 g/L). The standard simulation technique was employed for counter current extraction of metal ions from the brass pickle liquor. From the above solution copper was completely recovered in 4 counter-current extraction stages with 30% Versatic 10 acid (Figure 13), at an equilibrium pH of 4.0 and O/A ratio of 1.2:1. At the end of fourth stage the loaded organic contained 30.5 g/L Cu, and the raffinate contained 30 g/L Zn, 0.5 g/L Cr and 0.03 g/L Ni. In the loaded organic Zn, Ni and Cr contamination was not observed. From the raffinate chromium was removed as Cr(OH)3 by precipitation at pH 5.3. In this stage there was no loss of zinc with chromium precipitation. After removal of chromium from the aqueous solution zinc was recovered in 3 counter-current extraction stages using 30% Versatic 10 acid at the equilibrium pH of 5.6 and phase ratio 2:1 (Figure.14). At the end of the 3rd stage loaded organic contained 20.92 g/L Zn and the final raffinate contained 0.02 g/L Ni. Nickel contamination of the organic phase was not noticed.
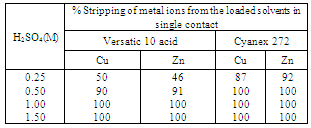 | Figure 13. Counter-current simulation for the extraction of copper. Aq. phase: 35 g/L Cu, 30 g/L Zn, 0.5 g/L Cr, 0.03 g/L Ni, Org. Phase: 30 % Versatic 10 acid in kerosene, O/A = 1.2:1, Eq. pH = 4.00 |
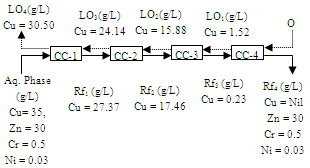 | Figure 14. Counter-current simulation for the extraction of zinc. Aq. phase: 30 g/L Zn, 0.03 g/L Ni, Org. Phase: 30 % Versatic 10 acid in kerosene, O/A = 2:1, Eq. pH = 5.6 |
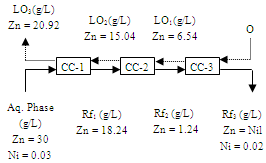 | Figure 15. Counter-current simulation for the extraction of zinc with Cyanex 272. Aq. phase: 35 g/L Cu 30 g/L Zn, 0.5 g/L Cr, 0.03 g/L Ni Org. Phase: 20% Cyanex 272in kerosene, O/A = 1.8:1, Eq. pH = 3.5 |
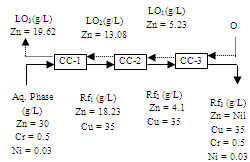 | Figure 16. Counter-current simulation for the extraction of copper with Cyanex 272. Aq. phase: 35 g/L Cu, 0.5 g/L Cr, 0.03 g/L Ni, Org. Phase: 20% Cyanex 272in kerosene, O/A = 1.8:1,Eq. pH = 4.6 |
4. Conclusions
- The recovery of copper and zinc from acid and iron depleted-spent brass pickle liquor by solvent extraction using Versatic 10 acid and Cyanex 272 as extractants in kerosene was studied. With Versatic 10 acid copper was extracted in the equilibrium pH range 3.28 to 4.9, whereas zinc was extracted in the pH range 5.1 to 7. The McCabe Thiele plot indicated that 4 counter current stages were required for complete extraction of copper and 3 counter-current stages for that of zinc with 30% Versatic 10 acid at the phase ratios of 1.1:1 and 1.9:1 at the equilibrium pH 4.0 and 5.6 respectively. Simulation of counter current extraction showed that at the end of fourth stage the organic phase was loaded with 30.5 g/L copper with no contamination with other metal ions present in the brass pickle liquor. After recovery of copper, zinc was completely recovered in three counter current extraction stages loading ~ 21 g/L Zn in the organic phase.Solvent extraction of copper and zinc, with a view to recover these metal ions from spent brass pickle liquor, was also studied using Cyanex 272 as an extractant in kerosene. Unlike Versatic 10 acid, Cyanex 272 extracted zinc at lower pH than copper. The difference in pH0.5 values for zinc and copper of 1.1 indicated better selectivity of zinc over copper. McCabe Thiele plot showed that zinc and copper could be recovered in three counter current extraction stages each. Stripping of copper and zinc from loaded organic was also studied. It was found that from loaded Versatic 10 acid both copper and zinc could be quantitatively stripped with 1 M sulfuric acid in a single stage whereas, from the loaded Cyanex 272 these metal ions were stripped quantitatively with 0.5 M sulfuric acid in a single stage. Comparison of extractability of Versatic 10 acid and Cyanex 272 shows that both the extractants can be effectively used for the extraction and separation of copper and zinc from the spent brass pickle liquor. The pregnant solution of copper and zinc can be processed to recover the metals in desired form. Thus, the present investigation opens up a scope to recover and recycle the metals from the brass pickle liquor in an eco-friendly manner.
ACKNOWLEDGEMENTS
- The authors are thankful to Director, CSIR-National Metallurgical Laboratory, Jamshedpur for giving permission to publish the paper. Thanks are also due to the Department of Science and Technology, Govt. of India for providing financial support under the DST – RFBR scheme.
References
| [1] | Kumar, V., Pandey, B.D., Bagchi, D., Akerkar, D.D., 1989, Scope of using LIX 84 for separation of copper and zinc from complex sulphide solution. Proc ICBMT, pp. 495-500. |
| [2] | Pandey. B.D., Kumar, V., Bodas, M.G., Akerkar, D.D., 1986, Separation and recovery of copper and zinc by solvent extraction and electrowinning from sulphate leach liquor of complex sulphide ore. Proc NSST, pp. 136-139 |
| [3] | Kumar, V., Bagchi, D., Pandey, B.D., 1997, Separation of copper and zinc from complex sulphate solutions by using LIX 84. Scandinavian Journal of Metallurgy 26, pp.74-78. |
| [4] | Reddy, B.R., Priya, D.N., 2004, Solvent extraction of Ni(II) from sulphates with LIX 84I flowsheet for the separation of Cu(II), Ni(II), and Zn(II). Analytical Sciences, 20, pp.1737-1740. |
| [5] | Reddy, B.R., Priya, D.N., 2005, Process development for the separation of copper(II), nickel(II), and zinc(II) from sulphate solutions by solvent extraction. Separation and Purification Technology, 45, pp.163-167. |
| [6] | Zhuo-yue, Lan.,Yue-hua, Hu., Jian-she, Liu., Jun, Wang., 2005, Solvent extraction of copper and zinc from bioleaching solutions with LIX 984 and D2EHPA. Journal of Central South University Technology, 12(1), pp. 45-49. |
| [7] | Cheng, C.Y., 2000, Purification of synthetic laterite leach solution by solvent extraction using D2EHPA. Hydrometallurgy, 35(3), pp.369-386. |
| [8] | Sole, K.C., Hiskey, J.B., 1995, Solvent extraction of copper by Cyanex 272, Cyanex 302 and Cyanex 301. Hydrometallurgy, 37, pp.129-147. |
| [9] | Tait, B.K., 1992, The extraction of some base metal ions by Cyanex 301, Cyanex 302 and their binary extractant mixtures with Aliquat 336. Solvent Extraction and Ion Exchange, 10, pp.799-809. |
| [10] | Kongolo, K., Mwema, M.D., Banza, A.N. Gock, E., 2003, Cobalt and zinc recovery from copper sulphate solution by solvent extraction. Minerals Engineering, 16, pp.1371-1374. |
| [11] | Owusu, G., 1999, Selective extraction of copper from acidic zinc sulfate leach solution using LIX 622. Hydrometallurgy, 51, pp.1-8. |
| [12] | Preston, J.S., 1985, Selective solvent extraction using organophosphorus and carboxylic acids and a non-chelating aldehyde oxime. United States Patent 4528167. |
| [13] | Dukov, I.L., Guy, S., 1982, Solvent extraction of zinc(II) and copper(II) with mixtures of LIX 34 and Versatic 911 in kerosene. Hydrometallurgy, 8, pp.77-82. |
| [14] | Preston, J. S., 1985, Solvent extraction of metals by carboxylic acids, Hydrometallurgy, 14, pp. 171-188. |
| [15] | Mahmoud, M.H.H., Barakat, M.A., 2001, Utilization of spent copper-pickle liquor for recovery of metal values. Renewable Energy, 23, pp.651-662. |
| [16] | Sastre, A.M., Miralles, N., Figuerola, E., 1990, Extraction of divalent metals with bis(2,4,4-trimethylpentyl) phosphinic acid. Solvent Extraction and Ion Exchange, 8, 597-614. |
| [17] | Sole, K.C., Hiskey, J.B., 1992, Solvent extraction characteristics of thiosubstituted organophosphinic acid extractants. Hydrometallurgy, 30, pp.345-365. |
| [18] | Agrawal, A., Kumar, V., Pandey, B.D., Sahu, K.K., 2006, A comprehensive review on the hydrometallurgical process for the production of nickel and copper powders by hydrogen reduction. Materials Research Bulletin, 41. pp. 879-892. |
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-Text HTML
Full-Text HTML