-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
International Journal of Materials Engineering
p-ISSN: 2166-5389 e-ISSN: 2166-5400
2024; 14(1): 12-24
doi:10.5923/j.ijme.20241401.02
Received: Jan. 22, 2024; Accepted: Feb. 17, 2024; Published: Feb. 29, 2024

A Review of Design and Development of Selected Transitional Metal Doped Zinc Sulphide Nanostructure Surface Layers Decorated with Graphene for Hydrogen Production
Joan J. Kiptarus1, Kiptiemoi K. Korir2, David N. Githinji3, Henry K. Kiriamiti4
1Department of Mechanical Production & Energy Engineering, Moi University, Eldoret, Kenya
2Department of Mathematics and Physics, Moi University, Eldoret, Kenya
3Department of Manufacturing, Textile and Industrial Engineering, Moi University, Eldoret, Kenya
4Department of Chemical and Processing Engineering, Moi University, Eldoret, Kenya
Correspondence to: Joan J. Kiptarus, Department of Mechanical Production & Energy Engineering, Moi University, Eldoret, Kenya.
| Email: |  |
Copyright © 2024 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Zinc Sulfide Nanostructures (ZnS NSs) have been proposed as potential photo-anode materials for Photoelectrochemical (PEC) water splitting due to their low toxicity, simple synthesis and easy modification routes. However, ZnS suffers from low PEC activity and photo-corrosion effects and therefore, application of ZnS NSs in PEC water splitting still awaits development of effective design and synthesis strategies to improve its PEC efficiencies to commercially viable levels. Given that most water splitting catalysts now on the market are made of expensive noble metals (such as ruthenium, iridium, rhodium, and platinum), there is a dire need for catalysts made of elementally abundant and less expensive elements. The general overview of ZnS Nanostructure layers for photocatalytic water splitting that employ selected transitional metal dopants (TMD) and include Graphene as a decorator for enhanced water splitting are discussed. This review provides a critical step toward the development of TM Doped ZnS NSs decorated with graphene for high photoelectrochemical (PEC) activity, especially in visible light.
Keywords: ZnS NSs, PEC, Photo catalysis, TMD, Graphene, Water splitting, Hydrogen
Cite this paper: Joan J. Kiptarus, Kiptiemoi K. Korir, David N. Githinji, Henry K. Kiriamiti, A Review of Design and Development of Selected Transitional Metal Doped Zinc Sulphide Nanostructure Surface Layers Decorated with Graphene for Hydrogen Production, International Journal of Materials Engineering , Vol. 14 No. 1, 2024, pp. 12-24. doi: 10.5923/j.ijme.20241401.02.
Article Outline
1. Introduction
- The global energy supply and related environmental issues are among the biggest technological challenges being confronted by chemists, technologists and engineers in the 21st century Arutyunov and Lisichkin [2017], Aithal and Aithal [2016]. Two of the most pressing problems facing the globe currently are global warming and the energy crisis; Sustainable and carbon neutral sources may be promoted as alternatives to fossil fuels Richardson et al. [2009], Peter [2018]. Natural resources such as coal and petroleum products as a source of energy are nearly exhausted and it is predicted that oil sup plies will run out in 50 to 150 years Lewis and Nocera [2006]. Additionally, the use of fossil fuels has led to a number of environmental issues, including rising emissions of greenhouse gases, most notably carbon dioxide CO2, NO2 and other air pollutants Netz et al. [2007] which has caused swerve climate change Ahmad et al. [2015], Fajrina and Tahir [2019], Faunce et al. [2013]. The total amount of energy consumed worldwide in 2008 was 15TW Petroleum [2009] and by 2050, it is predicted that this figure or the burn-rate of energy, will have roughly doubled due to the growing global production and population Du and Eisenberg [2012]. On that account, exploring renewable, clean and carbon-neutral alternative energy resources is desperately desired to limit our dependency on fossil fuels Quan et al. [2007]. The hunt for new renewable and sustainable energy sources sparked by rising energy consumption demand continuously presses for the creation of novel materials to address these issues as well as offering high alternative energy harvesting and storage capacities Shang et al. [2023], Sathre et al. [2016]. With over 60% of all global greenhouse gas emissions coming from energy, the United Nations (UN) claims that it is the primary cause of climate change. As a result, one of the 17 Sustainable Development Goals adopted by the UN also calls for the exploration of new renewable energy sources (Goal 7) Mai et al. [2022]. Therefore, it is believed that creating alternative energy sources with no carbon trace is essential for the survival of the world Korir et al. [2021]. Hydrogen presents itself as a potential alternative to carboniferous fossil fuels but only with consideration of an appropriate source. Considering all of the renewable sources (solar energy, wind, geothermal heat, tides, biomass, et al), solar energy is the most feasible and from a primary scientific view point, the most attractive alternative energy source Esswein and Nocera [2007]. Due to the major environmental consequences of the depletion of fossil fuels, there is growing interest in the conversion of water and solar energy into clean and sustainable H2 fuels utilizing elements that are abundant on earth Li et al. [2015]. Hydrogen is one such fuel with no emission of pollutants when burned in oxygen Li et al. [2023], Hassan et al. [2023a], it is the most abundant and cleanest element in nature and has the highest energy content in common fuels which is almost three times that of gasoline in the same weight Bellani et al. [2019]. As an ideal energy carrier, hydrogen can be used in various fields such as, communications and transportation, electric power, architecture, industry, in vehicles, spacecraft propulsion, aircraft and electric devices Joy et al. [2018]. By using a hydrogen-oxygen fuel cell, hydrogen can be converted into electricity and the byproduct of this process is only water, thus used as a renewable energy. So far, the main ways to obtain hydrogen are by reforming the natural gas, coal, oil, but these approaches usually come along with the emission of carbon dioxides which do not meet the vision of zero carbon emission Yu et al. [2021], Bauer et al. [2022], Liu et al. [2022]. Scientists hypothesized that using solar, wind, hydro, geothermal and nuclear energy as power source to produce hydrogen will realize a real zero carbon source of hydrogen production Boretti [2023], Turner [2004]. Future alternatives to fossil fuels that are considered are hydrogen and electric power. However, there remain significant obstacles impeding the growth of hydrogen energy, regardless of how hydrogen is produced, stored, transported, or used Chen et al. [2021a]. The quest for a clean, renewable and sustainable energy future has been highly sought for by the scientific community over the last four decades.
2. Photocatalytic Water Splitting
- Photocatalytic water splitting is expected to become the dominant method of hydrogen production as a promising approach for clean, economical and environmentally friendly production of hydrogen by using solar energy since Fujishima and Honda first reported the photoelectrochemical water splitting on a TiO2 electrode in 1972 Gopinath and Nalajala [2021], Fujishima and Honda [1972]. The relative high energy barrier and slow kinetic properties require utilization of catalyst to improve this situation. At present, noble metal-based materials including ruthenium, iridium, rhodium and platinum are the state-of-the-art water splitting electro catalysts due to their high reactive in acidic and alkaline condition Wang et al. [2011], Li et al. [2020], Cavaliere [2023]. However, the cost of using these catalysts on a wide scale has significantly increased by their rarity and the mining whereby the refining procedures have a negative impact on the environment. Exploring a reliable and stable non-noble metal-based material is therefore the focus of many research efforts today Chen et al. [2021a]. Energy disciplines have increasingly put much interest in to transition metal base materials such carbides, nitrides, phosphides, sulfides, oxides and their composites Voiry et al. [2013], Wang et al. [2020a], Zhao et al. [2020]. Transition metal base materials offer a wide range of applications in areas including energy storage and conversion, sensors and photo or electro catalysis due to their distinctive electrical structure and great physical properties Wang et al. [2016], Patel et al. [2018]. Nanostructures materials have become the main attraction for material researchers due to their contributions to different disciplines such as environment, electronics, photonics, medicine, etc. Various kinds of nanomaterials have been developed and are currently utilized in innumerable applications Razali and Ismail [2021]. Due to their distinctive chemical and electrical structure, ZnS, a group II–VI semiconductor, has attracted a lot of interest because it has proven to be an effective photocatalyst Agorku et al. [2015]. The large band gap (3.60 eV) prevents it from being utilized for photo catalysis in the visible light range. To fully utilize the potential of sunlight for hydrogen synthesis, a photocatalyst material with visible-light activity must be used Kurnia et al. [2016], Wang et al. [2020b], Villa et al. [2021], Hao et al. [2018], Fajrina and Tahir [2019].
2.1. Designing Efficient Photocatalytic Material for Hydrogen Production
- The process of designing visible light driven photo catalysts begins with band engineering. It is well acknowledged that a material’s performance in photo electrochemical (PEC) and other optoelectronic applications is influenced by its structure and morphology, which include defects in both the bulk and sur face, as well as particle size, crystal structure and surface morphology. Lee and Wu [2017], Kong et al. [2011], Bell et al. [2011]. Photoelectrochemical (PEC) catalysis and electro catalysis are some of the approaches of hydrogen fuel production via water splitting. PEC water splitting based devices utilize an active semiconductor anode material such as ZnS to absorb solar energy necessary to break the chemical bond of a water molecule. The oxygen evolution reaction (OER) and the hydrogen evolution reaction (HER) are the two chemical processes of water splitting Nellist [2016]. As seen in Figure 1, these processes don’t have favorable thermodynamics hence, an external potential bias is needed to cause the uphill response ∆E0 = 1.23V. The OER takes place in electrolytic cells and involves four electron transfers (see equation 1) and is viewed as the challenge in breaking down water molecules into H2 and O2 gases.
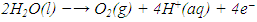 | (1) |
 | Figure 1. Schematic diagram of an electrochemical hydrogen and oxygen reaction. (Source; Zhu et al. [2019]) |
2.2. Semiconductor Metal Sulphide
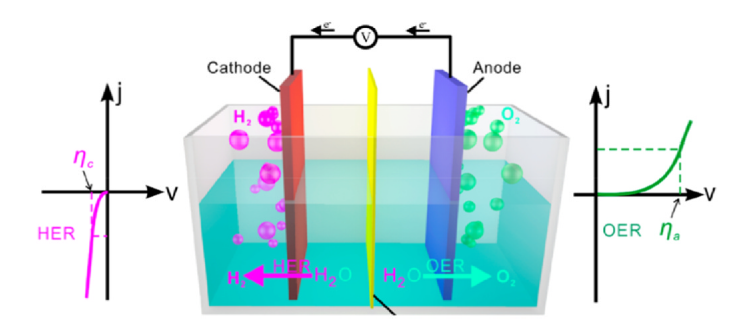
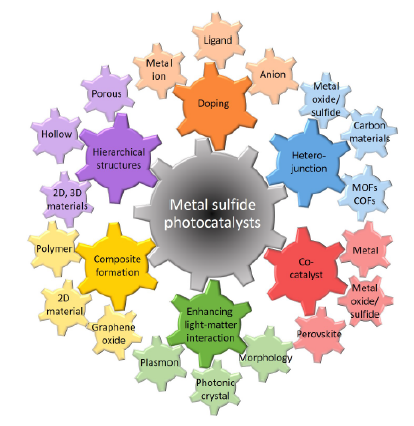
- Fujishima and Honda’s pioneering work from 1972 Fujishima and Honda [1972] documented water splitting for H2 production over TiO2 semi-conductor. Since then, numerous semiconductor kinds have been studied for photocatalytic H2 production. Multiple active photocatalysts including different oxide, sulfide, and oxy-nitride semiconductors have been developed for the afore- mentioned photocatalytic reaction Zhang et al. [2012a]. Because of their ad- equate band gap and catalytic function, metal sulfides and their composites are among them and are considered ideal candidates for photocatalytic H2 production. Recent years have seen a lot of interest in the exceptional physical and chemical properties of semiconductor metal sulfides. Very lately a number of binary metal sulfide (MS) compounds, including PbS, CdS, ZnS, MoS2, SnS2, Bi2S3, In2S3, Cu2S, NiS/NiS2 and CoS2 as well as their derivatives and heterostructures, have been extensively utilized for a variety of uses Maeda et al. [2006], Bonde et al. [2009], Balayeva and Mamiyev [2016], Baran et al. [2015]. The most popular metals used in photocatalytic hydrogen production as sulfide compounds have been summarized in figure 2. The majority of these compounds have been found to exhibit a remarkable reactivity to visible light, enough active sites and the right reduction potential of H+/H2 to function as an efficient photocatalyst. Additionally, new quantum size effects allow for further tuning towards quick charge transfer, longer excited state lifetimes Keimer and Moore [2017], Frame and Osterloh [2010]. Therefore, a lot of effort has been put towards enhancing solar hydro- gen generation over MS semiconductor photocatalysts over the past ten years Sivula and Van De Krol [2016], Amirav and Alivisatos [2010]. Among other Metal sulfide (MS) materials ternary sulfides, such as CuInS2 and CuGaS2, exhibit good selectivity and a high rate of photocatalytic generation, especially when combined with titanium dioxide TiO2 Shiga et al. [2016], Hou et al. [2020].
 | Figure 2. Chemical elements of metal sulfide compounds for photocatalytic hydrogen production (Source; Maeda et al. [2006]) |
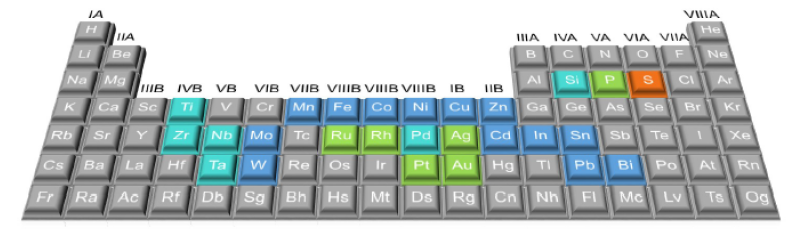
 | Figure 3. General requirements for designing high efficiency photocatalysts. (Source; Chen et al. [2021b]) |
2.3. Transitional Metal Doping of ZnS Nanostructure Layers
- Doping is necessary in order to modify the intrinsic semiconductors properties. An average of one dopant atom for every 105 atoms is found in the doping concentration Pereira [2017], Kripal et al. [2010]. The intermediate gap states created by surface species are transferred outside the gap area to generate impurity centers when impurities, electrons, and holes are doped into semiconductors Zunger and Malyi [2021]. Doping has no effect on the absorption of a spectrum, but it greatly increases the intensity of its emission Yang et al. [2019]. The process mixture is simply mixed with the dopant to achieve the desired doping. If, as in the case of rare earth elements and transition metals, the impurity-induced transition is bounded, then the radiative efficiency of the impurity-induced emission increases considerably. Consequently, doped nanomaterials bring about significant advancements in the domains of optics, photonics, electronics, medicine, biosensors, solar cells, IR detectors, color television, phosphors, light emitting diodes, field emitting diodes, lasers, photocatalysts, photo detectors, and electroluminescence sensors (ELS) Chen et al. [2005], Zhang et al. [2020], Palani et al. [2022], Rahimi- Iman [2020]. Because of their high surface to volume ratio, ZnS nanoparticles exhibit a considerable band gap, which makes them more promising carriers of photoluminescence Bhargava et al. [1994]. ZnS nanoparticles with different chemical and physical properties can be produced by doping; this is required to use semiconductor nanocrystals for the production and sale of nanoscale devices. The impurity centers that interact with electrons and holes are created by dopants. In this regard, a number of researchers have attempted to build semiconductor nanostructures doped with transition metal Choi et al. [2005], Radovanovic et al. [2005], Yatsunenko et al. [2008]. Since ZnS has a large band gap (3.6 eV), it can only absorb in the UV, but it is easy to change its absorbance by adding metal ions like Mn, Ni, Cu, or Pb Kudo and Sekizawa [2000], Tsuji and Kudo [2003]. Significant progress has recently been achieved in the production of transition metal doped III-V and II-VI semiconductor nanostructures, since theoretical research shows that these materials could exhibit high Curie temperatures Dietl et al. [2000]. By moving the center of recombination away from surface states and toward impurity states, impurities improve radiative efficiency. ZnS nanoparticles doped with transition metal ions are the most important material for studying semiconductor nanocrystals; they also hint at a new class of illuminating materials. The electronic configuration of the Mn+2 ion, for example, is [Ar]3d5. Quantum efficiency is increased because the energy transition between the ZnS lattice’s s-p states and these Mn+2 d states happens more quickly because of their hybridization. Numerous studies Bol and Meijerink [1998], Li et al. [2023, 2015, 2008] indicate that the broad emission peak of the Mn+2 ions is influenced by the host lattice. Moreover, doping various optically active luminous materials has been shown to enhance a number of properties, most notably an increase in emission intensity throughout a wide wavelength range. Among the reported dopants, Cu doping has attracted the most attention because of its strong concentration dependency Lee et al. [2008]; this attracts the different energy levels, producing luminous spectra. In earlier research, ZnS doped with Cu, Ni, and Pb has been examined for the production of photocatalytic H2 Arai et al. [2008], Yu et al. [2007], Liu [2010]. Cu doped ZnS was found to have a low chemical stability but a high quantum efficiency of up to 3.7% at 420 nm Kudo and Sekizawa [2000]. Although Ni-doped ZnS has less activity for generating H2, its quantum efficiency was found to be 1.3% at 420 nm and 2.1% at 430 nm by Bang et al. [2008]. Compared to Cu and Ni doped ZnS, Pb-doped ZnS exhibited substantially poorer photocatalytic activity. ZnS only absorbs in the UV on its own, thus altering its absorbance by doping it with specific transitional metal (TM) ions is simple Devi and Kavitha [2013], Mahmoud et al. [2018], Jothibas et al. [2018]. The ZnS absorption edge may move in this way, moving into the visible region, and new energy levels may be created between the ZnS valence band maximum (VBM) and conduction band minimum (CBM) Belver et al. [2019], Bai et al. [2018], Khaki et al. [2017], Kurnia et al. [2016], Fadojutimi et al. [2022]. As a result, doping may induce structural defects which can successfully modify band structure and control photoactivity. Therefore, TM dopants may enhance charge separation, reduce band gap and photo-corrosion impact and show noticeable light absorption photo-activity Korir et al. [2021] and can also increase PEC application performance.
2.4. Effects of Graphene on ZnS Nanostructure Layers
- Improving charge separation and transfer, promoting optical absorption, maximizing band gap position, reducing cost and toxicity, and boosting stability and water splitting kinetics are the main effects of graphene which explains the advantage of how well heterogeneous semiconductors can split water using solar Razaq et al. [2022]. A greater possibility of developing extremely effective photo catalysts appears to exist when various engineering procedures are combined, particularly when co-catalyst loading and other strategies are used Ray [2015], Rowley-Neale et al. [2018]. To determine the key efficiency limiting phase and develop highly efficient solar-to-fuel conversion systems, a comprehensive grasp of the fundamentals of electron and hole transfer thermodynamics and kinetics is essential Prasad et al. [2020]. The combination of graphene and other inorganic functional materials holds great promise for many potential applications, including energy storage materials, Nano-electronic devices, and photovoltaics, given the notable physical, chemical, and mechanical properties resulting from the two dimensional (2D) form of graphene sheets Xu et al. [2013], Khan et al. [2020]. The network of conjugated sp2 bonds in graphitic carbon results in a greater number of delocalized electrons, which in turn increases the transit of electrons optically created in semiconductor particles and so increases the photo conversion efficiency of the system. Typically, reduced graphene oxide (rGO) is synthesized from graphene oxide (GO) to create graphene-based metal or semiconductor nanocomposites Vivekchand et al. [2008]. Xiang and Yu [2013] investigated the synthesis, evaluation, and possible uses of semiconductor and graphene-based photocatalysts in photocatalytic hydrogen production. This significantly improved the material’s photocatalytic performance by raising adsorption capacity, gathering visible light, and lowering band gap energy. In accordance with their band gap, semiconductor nanoparticles emit light that causes electrons and holes to form in the valence and conduction bands, respectively Kamat [2011], Zhang et al. [2012b]. Moreover, studies have demonstrated that this strategy can prevent the recombination of electron hole pairs generated during photo catalysis An and Jimmy [2011], Han et al. [2012]. A semiconductor photocatalyst with long lived charge carriers, fewer charge trapping centers, the right energy level offsets, and stability against light are all highly wanted for increasing photo catalytic reactivity Xiang and Yu [2013], Ahmed and Mohamed [2023]. Notwithstanding extensive research on the photocatalytic development studies based on graphene for high photocatalyst efficiency, there are still more research gaps and challenges that require careful attention in the future.
2.5. New Advancements in Hydrogen Production
- Despite being thought of as an economical, clean, sustainable, and environmentally helpful technology, the utility of MS semiconductor-based photocatalysis is limited, even though photo-excited electron hole pairs recombine quickly and require little solar energy there are several strategies that have been developed to overcome these innate limitations Hassan et al. [2023b], Xue et al. [2022] the optimization of the light spectrum absorbed by semi- conducting photocatalysts has recently progressed with the synthesis and decorating of novel particle morphologies. Phenolic structures, porous morphologies, 1D nanowires (NWs), 0D quantum dots (QDs), 2D sheets, and layered structures are among the materials currently being studied and developed for photocatalytic hydrogen generation; these materials are depicted in Figure 4. In particular, bandgap size reduction for MS enhances the contacting surface areas and inter-particle interactions, facilitating rapid photo-excited charge transfer.
 | Figure 4. Metal sulfide Strategies for hydrogen production, (Source; Hassan et al. [2023b]) |
2.6. Development Methods of Photocatalysts for Hydrogen Production
- Photo catalytic water splitting is a very promising technology to proffer a solution to present day environmental pollution and energy crises by generating hydrogen fuel through a “green route” without environmental pollution Fadojutimi et al. [2022]. In this sense, the focus of expanding basic and applied research has been on H2 production as a sustainable energy source. In general, multiple chemical methods can be used to obtain hydrogen from various sources, such as coal or liquid fossil fuels, as shown in Figure 5.
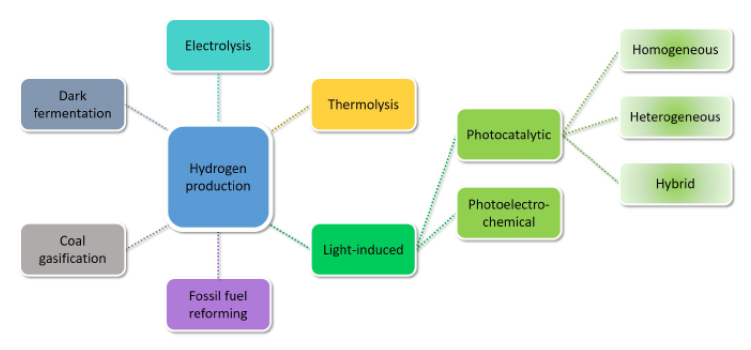 | Figure 5. Chemical methods to obtain hydrogen. (Source; Fadojutimi et al. [2022]) |
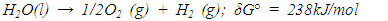 | (2) |
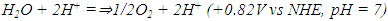 | (3) |
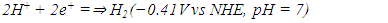 | (4) |
3. Conclusions
- The large-scale applications of photocatalytic platforms for efficient solar hydrogen H2 production have not yet been thoroughly explored, despite the fact that tremendous progress has been made in recent decades in the design of highly active photocatalysts, such as semiconductor bio-hybrids, organic semiconductors and plasmonic nanoparticles (NPs) Qi et al. [2011]. However, there aren’t many thorough studies that take into account a wide variety of TMD’s as a potential to fulfill future energy requirements for increased PEC water splitting. The density functional theory (DFT) framework Chaurasiya and Dixit [2019], Akhtar et al. [2017], implemented in the Quantum ESPRESSO package, should be used to investigate the structural, electronic and optical properties, as well as the chemical stability, of selected transitional metal doped ZnS NS layers. In order to achieve optimal hydrogen production, extensive experimental studies should also be carried out to evaluate the photocatalytic activity of different TM doped ZnS NS layers decorated with graphene. The surface morphologies and material properties of the nanostructures should also be investigated to analyze the broad range of material metrics, including particle size, crystal structure, surface morphology, chemical composition, and band gap by employing techniques such as; Raman spectroscopic (RS) microscopy, Fourier Transmission Infrared Spectroscopy (FTIR), X- ray photocurrent spectroscopy (XPS), scanning electron microscopy (SEM), and X-ray diffraction (XRD). Therefore, research on TM doped ZnS nanostructure photocatalysts decorated with Graphene is desperately needed to confirm the photocatalytic effectiveness of graphene based semiconductors in the production of hydrogen (H2) molecules for industrial applications.
ACKNOWLEDGEMENTS
- The authors acknowledge the African Development Bank (AFDB), the SAMat Research Facilities, Jawaharlal Nehru Centre for Advanced Scientific Research (JNCASR), Bengaluru, India, Center of Excellence in Phytochemicals, Textile and Renewable Energy (ACE II PTRE), Strengthening mobility and Promoting Regional integration in Engineering Education in Africa (SPREE) and Moi University, Kenya for support.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML