-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
International Journal of Materials Engineering
p-ISSN: 2166-5389 e-ISSN: 2166-5400
2023; 13(1): 1-3
doi:10.5923/j.ijme.20231301.01
Received: Nov. 12, 2023; Accepted: Nov. 25, 2023; Published: Dec. 13, 2023

Synthesis and Research of Biologically Active Compounds Based on Aminolupinin
Tureniyazova Dametken Ayniyazovna1, Kushiev Khabibjon Khozhiboboevich2
1Karakalpak State University, Nukus, Uzbekistan
2Gulistan State University, Gulistan, Uzbekistan
Correspondence to: Tureniyazova Dametken Ayniyazovna, Karakalpak State University, Nukus, Uzbekistan.
| Email: |  |
Copyright © 2023 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

The article presents the results of the synthesis and study of a number of lupinin derivatives with an amide bond to search among them for effective biologically active substances that have a certain superiority over known drugs.
Keywords: Quinolizidine alkaloids, Anabasis aphylla, Aral Sea region, Aminolupinine, Benzyl esters, Benzoylamidolupinin, Biological activity
Cite this paper: Tureniyazova Dametken Ayniyazovna, Kushiev Khabibjon Khozhiboboevich, Synthesis and Research of Biologically Active Compounds Based on Aminolupinin, International Journal of Materials Engineering , Vol. 13 No. 1, 2023, pp. 1-3. doi: 10.5923/j.ijme.20231301.01.
1. Introduction
- The creation of new biologically active substances and the study of the mechanisms of their action remains one of the most urgent tasks of modern bioorganic chemistry. Modification of natural compounds, especially alkaloids, is considered one of the priorities in its implementation. Modification of quinolizidine alkaloids opens up great prospects in the search for biologically active substances with high efficiency, selectivity and stereospecificity [1].Quinolizidine alkaloids attract the attention of researchers with their biological activity and structural diversity. In this regard, the alkaloids lupinin and its derivatives isolated from the plant Anabasis aphylla are of considerable practical interest. The poisonous and medicinal properties of this plant have been known since ancient times and it has been used in the treatment of wounds and various diseases, including tuberculosis. Decoctions of the plant were used to control pests of agricultural crops [1-3].Lupinin is the second alkaloid in terms of content in the plant Anabasis aphylla and a waste product in the production of anabasine hydrochloride. The availability of lupinin and the methods of its isolation are one of the advantages, which makes it possible to synthesize various biologically active substances with specified properties on its basis [1-3,9,10,11].Currently, there is a lot of factual material on the physiological activity of lupinin and its derivatives. The relatively easy availability of lupinin and the presence of a simple alcohol group in the structure of the quinolizidine system, which makes it easy to obtain various derivatives of this alkaloid, are the reason for the search for active drugs among the derivatives of this alkaloid.
2. Material and Methods
- The object of the study was the plants Anabasis aphylla, which grows in the Aral Sea region, the alkaloids lupinin isolated from the plant and its derivative aminolupinin. New modified derivatives of aminolupinin have been synthesized. The individuality of the synthesized compounds was controlled by thin-layer chromatography. The structure of the synthesized compounds was confirmed by the data of IR-, PMR- and mass spectra.
3. Results and Discussion
- In order to find new highly effective biologically active drugs among the derivatives of lupinin isolated from Anabasis aphylla plants, we synthesized a number of lupinin derivatives with substituted benzoic acids bound by an amide bond according to the following structure:
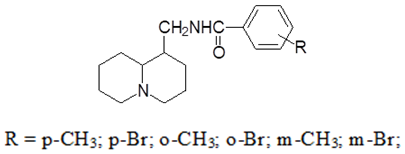 To achieve the maximum yield of the reaction product, a short-term heating of the mixture is required after the addition of reagents. The reaction can be carried out without triethylamine. In this case, the reaction mixture must be heated for more than three hours, after which, when potash is added, the base of the aminoester, substituted benzoylamides of lupinine is released from the hydrochloride. Of all the synthesized compounds, para-bromine- and methane-bromine-substituted benzoylamidolupinins (with prolonged standing, para-bromine- and meta-bromine- substituted benzoylamidolupinins crystallize) are oily, the rest are crystalline substances with a yellowish tinge. The synthesized compounds were purified by recrystallization from benzene. The chlorohydrates of the compounds were obtained by the action of dry hydrogen chloride on a solution of benzoylamidolupinins. All hydrochlorides are obtained clean and do not require further cleaning. The characteristics of the synthesized compounds are given in Table 1.
To achieve the maximum yield of the reaction product, a short-term heating of the mixture is required after the addition of reagents. The reaction can be carried out without triethylamine. In this case, the reaction mixture must be heated for more than three hours, after which, when potash is added, the base of the aminoester, substituted benzoylamides of lupinine is released from the hydrochloride. Of all the synthesized compounds, para-bromine- and methane-bromine-substituted benzoylamidolupinins (with prolonged standing, para-bromine- and meta-bromine- substituted benzoylamidolupinins crystallize) are oily, the rest are crystalline substances with a yellowish tinge. The synthesized compounds were purified by recrystallization from benzene. The chlorohydrates of the compounds were obtained by the action of dry hydrogen chloride on a solution of benzoylamidolupinins. All hydrochlorides are obtained clean and do not require further cleaning. The characteristics of the synthesized compounds are given in Table 1.
|
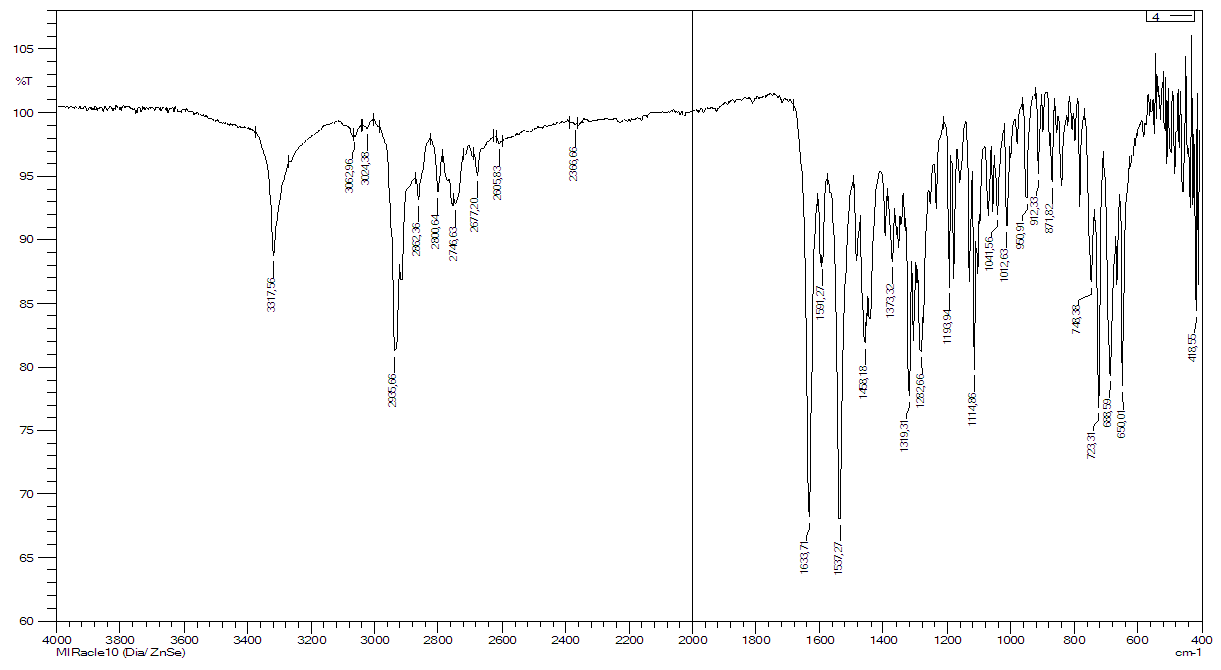 | Figure 1. IR spectrum of 2-methylbenzoylamidolupinin (III) |
4. Conclusions
- Modified derivatives of lupinine with an amide bond have been synthesized on the basis of aminolupinine with chlorohydrides of substituted benzoic acids. Based on a comprehensive physico-chemical study of synthesized compounds, their structure has been established. All the obtained spectra confirm the structure of synthesized benzyl esters of aminolupinin.The study of the biological properties of synthesized compounds can lead to the creation of new effective drugs that have a certain superiority with known drugs in medicine or in agriculture to increase the yield and quality of crops.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML