-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
International Journal of Materials Engineering
p-ISSN: 2166-5389 e-ISSN: 2166-5400
2016; 6(5): 166-172
doi:10.5923/j.ijme.20160605.05

Zinc Oxide Nanoparticles Synthesized by Microwave-Assisted Combustion Method and Their Potential for Ethonal Gas Detection
L. C. Nehru1, C. Sanjeeviraja2
1Department of Medical Physics, School of Physics, Bharathidasan University, Tiruchirappalli, India
2Department of Physics, Alagappa Chettiar College of Engineering & Technology, Karaikudi, India
Correspondence to: L. C. Nehru, Department of Medical Physics, School of Physics, Bharathidasan University, Tiruchirappalli, India.
| Email: |  |
Copyright © 2016 Scientific & Academic Publishing. All Rights Reserved.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

A facile and rapid microwave-assisted combustion method (MWAC) was developed to synthesis nanocrystalline ZnO powder using dissolution of zinc nitrate (as the oxidant) and glycine (as fuel) as the starting materials and water as solvent, then heating the resulting solution in a microwave oven. The study suggested that application of microwave heating to produce the homogeneous porous ZnO was achieved in few minutes. The starting materials of the combustion product and the influence of the different calcination temperature was discussed based on thermogravimetric analysis and X-ray powder diffraction (XRD). The structure and morphology of the as-prepared and annealed combustion products was investigated by means of XRD and SEM. The average particle size of the nanoparticles synthesized at as-prepared, 400, 500 and 600°C was calculated to be 20, 27, 29 and 40 nm, respectively. Thick-film sensor samples based on the as-synthesized ZnO nanoparticles without specific additives showed response sensitivity of around 18.05 at the optimal detection temperature of 150°C for 25 ppm C2H5OH gas and recovery times were quite fast, of the orders of seconds. The detection limit is lower than the limit needed for breath analyser and the fast dynamics are compatible with a real application also if response to possible interfering gases still have to be investigated.
Keywords: Nanocrystalline, ZnO, Combustion method, Gas sensor
Cite this paper: L. C. Nehru, C. Sanjeeviraja, Zinc Oxide Nanoparticles Synthesized by Microwave-Assisted Combustion Method and Their Potential for Ethonal Gas Detection, International Journal of Materials Engineering , Vol. 6 No. 5, 2016, pp. 166-172. doi: 10.5923/j.ijme.20160605.05.
Article Outline
1. Introduction
- The fabrication of transition metal oxide semiconductors with nano structure has been the target of scientific interests for gas sensors such as CO, H2, H2S, C3H8, C2H6O, CH3COCH3, NO and NO2 [1, 2]. However, these sensing materials can react with several gases simultaneously, and their sensing characteristics (e.g., response, recovery, reproducibility, stability, and linearity) have yet to be improved. Among these oxides, the study of zinc oxide (ZnO) nanostructure is paying considerable attention in many areas of chemistry, physics and material science, mainly due to its broad applications in gas leak detection and environmental monitoring. At 200–400°C, a resistive electron depletion layer is formed near the surface of the n-type oxide semiconductor by the chemisorption of ambient oxygen. In addition, the interaction between negatively charged surface oxygen and target gases induce large changes in electrical conductivity [3, 4]. Because of high surface-to-volume ratio in gas sensing, the fabrication of a well-defined nano powder has been the main research approach over the past three decades. However, because many types of reducing and oxidizing gases can interact with the sensing material simultaneously, the selective sensing of a specific gas is not easy to accomplish. The main advantages are the simple sensor structure and operating algorithm. However, these sensing materials react with several gases simultaneously. Various approaches have been introduced to achieve selective gas sensing. Due to the several advantages of ZnO in pure and doped nanomaterials, it gained much importance to study using various methods and their ethanol gas sensing properties [5-11].However, these methods have some disadvantages, such as complex operating procedures and the high price of raw materials. Furthermore, they generally need high-temperature treatment. The gas-sensing properties were related to the synthesis method and preparation condition, so the response and the detection limit could be improved by changing the synthesis method and optimizing the preparation condition. Many authors proved that the sintering of nanoparticles under standard conditions used for micron-sized particles led to a dramatic growth of particles and to a loss of the nanostructure in the sintered sample. Microwave irradiation as a heating method has found a number of applications in chemistry. The utilization of microwave irradiation in the preparation of nano particles have been reported in recent years. Compared to the conventional methods, the microwave synthesis have the advantages of producing small particle size metal oxides with high purity owing to short reaction time, enhanced reaction selectivity, and energy saving [12]. Recently, microwave-assisted combustion synthesis is being acknowledged as a novel method to produce materials with an efficient, cost-effective, fast, mild, energy-efficient and environmentally friendly rout for easy synthesis of nanosize oxide powders. Since microwave heating is an in situ mode of energy conversion and the microwave heating process is fundamentally different from conventional heating processes. Heat will be generated internally within the material, instead of originating from external sources. However, there are few reports available using microwave-assisted combustion synthesis method will also be useful for discovering new gas sensor materials as well as in establishing material libraries for gas sensing. The aim of the present work is to synthesize and characterize the microwave-assisted combustion synthesis method prepared ZnO nanoparticles using glycine as fuel and to investigate the ethanol sensing properties of as-synthesized and different calcination temperature of ZnO nanoparticles.
2. Experimental
2.1. Preparation of ZnO Nanoparticles
- All the reagents used in the experiments were analytically pure and were purchased from MERCK Company, and were used as received without further purification. Nanocrystalline ZnO was synthesized by a microwave-assisted combustion synthesis process using glycine as fuel. A schematic representation of the synthesis process used in the current study is graphically shown in Fig. 1. Stoichiometric amounts of zinc nitrate and glycine were dissolved in deionized water and transferred into a quartz container for mixing well by magnetic stirring for 1/2 h, to make them almost as homogeneous mixture, which further was placed in a domestic microwaveoven (BPL India Limited, Bangalore, India, Model No. IFB, 17PG1S, microwave 700 W, input range 210–230 V-ac 50 Hz, microwave frequency 2.45 GHz). Initially, the solution boils and undergoes dehydration followed by decomposition with the evolution of large amount of gases with white fumes coming out from the exhaust opening provided on the top of the micro oven. After reaching the point of spontaneous combustion in the solution it begins burning and releases lots of heat, vaporizes all the solution instantly and becomes a foamy white solid powder.
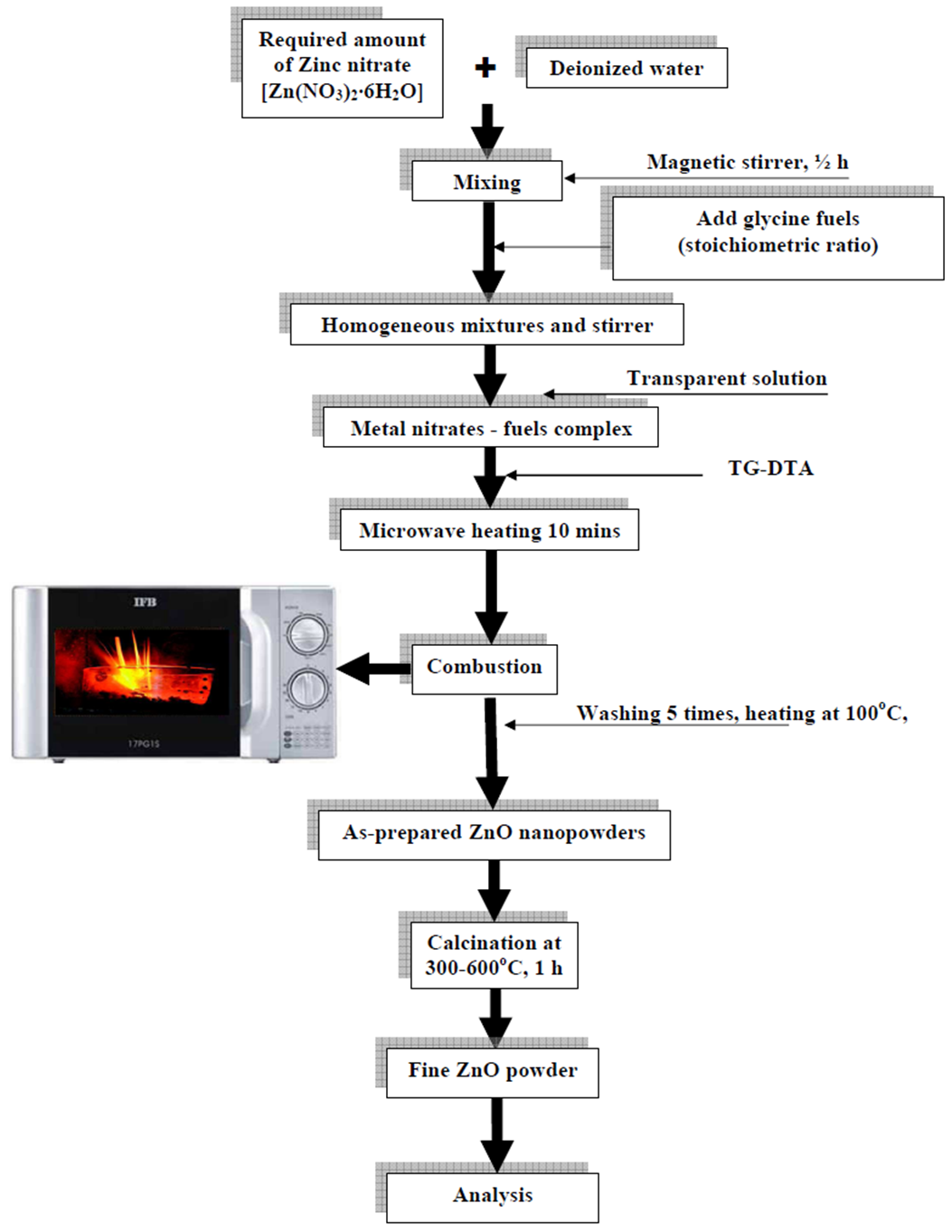 | Figure 1. Schematic representation of the synthesis process |
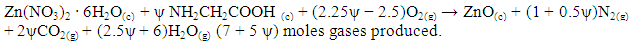 | (1) |
2.2. Formation of Thick-Film Sensors
- All the sensor samples was fabricated by standard screenprinting thick-film technology. As-prepared ZnO nano powder was mixed and ground with an adhesive in an agate mortar with an aqueous solution of PVA (polyvinyl alcohol) to form a gas-sensing paste. A small amount of zinc powder and glass frit was added in order to improve the film strength and the adhesion to the substrate. The resulting pastes directly coated on alumina substrate which is used to study the ethanol gas sensing properties. The films were then dried at 100°C for 1 h, to remove PVA in the coated layer. In this screenprinting method, we developed thick films to form a large scale on the substrate and this approach is more reproducible, cost-effective and higher yield process. Electrical contacts were made with copper wire on either side of the thick film sample coated on alumina substract. The central part of the film is exposed to the gas medium. The sizes of the thick films area are 1 x 1 cm2 and the active part of the sensor area exposed to the ethanol gas was 0.8 x 0.8 cm2. The thickness of the prepared thick films was about 6 to 8 μm.
2.3. Characterization
- The metal nitrates - fuels complex sample after drying at 80°C in air for 1/2 h was subjected to thermogravimetric and differential thermogravimetric analysis (TG–DTG) using a Perkin Elmer, Norwalk, CT, USA at a heating rate of 10°C/min in static air. The synthesized ZnO powders identified the phase formation by powder X-ray diffraction (PXRD) method using a X’ Pert PRO PANalytical diffractometer using nickel filtered Cu-Kα radiation (λ=0.15418 nm) as source and operated at 40 kV and 30 mA. The sample was scanned in the 2θ ranging from 10 to 80° in the step scan mode. The observed peak positions were compared with the standard ICDD data and Miller indices were assigned to the Bragg peaks. Morphology of the materials was observed by scanning electron microscope (SEM) (Hitachi S4800). SEM measurements were mounted on aluminum studs using adhesive graphite tape and sputter coated with gold before analysis.
2.4. Experimental Set-up and Measurements of Gas-Sensing Properties
- The gas-sensing response of the as-prepared ZnO nano powder was characterized by gas-sensing characterization as shown in Fig. 2, where the sensor samples were mounted in a special home made designed gas testing vacuum chamber with controlled temperature and humidity.
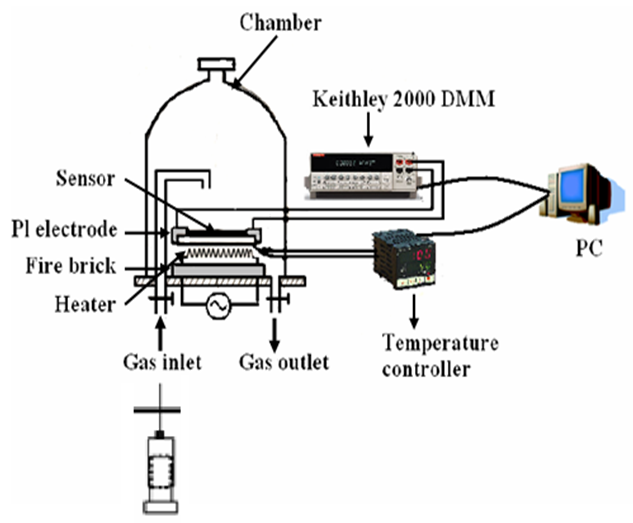 | Figure 2. The schematic diagram of the gas sensor measurement setup |
3. Results and Discussion
3.1. Pyrolysis of the Gel
- The Figure 3 shows the thermogravimetric and differential thermogravimetric (TG–DTG) curves of the metal nitrates - fuels complex sample after drying at 80°C in air for 1/2 h.
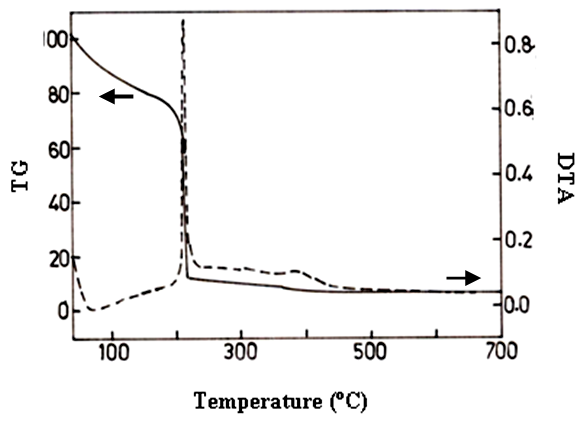 | Figure 3. TG/DTA cure of glycine mixed Zn(NO3)2 |
3.2. Microstructure Analysis
- The XRD patterns of powders synthesized at various temperatures of 400–600°C are shown in Fig. 4. It is indicated with the reflecting planes (1 0 0), (0 0 2), (1 0 1), (1 0 2), (1 1 0), (1 0 3), (2 0 0), (1 1 2) and (2 0 1) in the patterns. All the 2θ values 31.64, 34.30, 36.13, 47.42, 56.47, 62.74, 66.27, 67.82 and 68.97º were well consistent with ASTM (card number 04-008-8198) and no diffraction peaks corresponding to Zn and other impurities were observed, which confirmed the samples as a pure hexagonal wurtzite crystalline ZnO. The value of lattice parameters was calculated by the diffraction angles using the method described elsewhere [13], this results are good agreement with the standard diffraction patterns. It can be seen that the XRD major peaks was broadened. From the particle size measurements, the average particle sizes of ZnO nanoparticles synthesized at as-prepared, 400, 500 and 600°C was calculated to be 20, 27, 29 and 40 nm, respectively. For the particles synthesized at higher calcination temperatures, XRD peaks became sharper without obvious extension in width. It can be concluded that crystallinity increased with the calcination temperature in the range of 400–600°C.
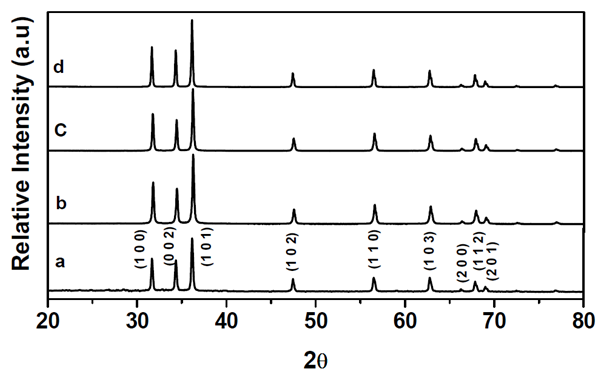 | Figure 4. XRD patterns of ZnO synthesized by a microwave-assisted combustion method. (a) as-prepared, (b) calcination temperature at 400°C, (c) 500 and (d) 600°C |
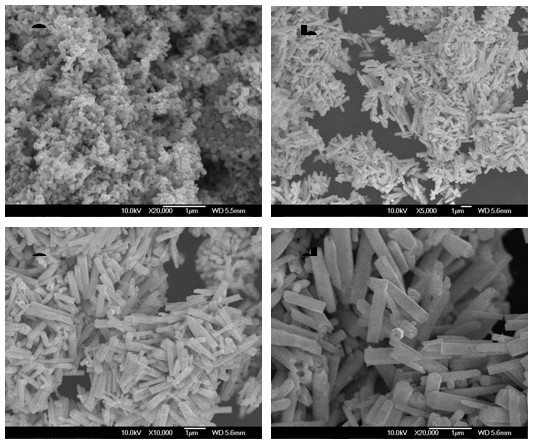 | Figure 5. SEM image of glycine used ZnO nanoparticles with different calcination temperature at (a) as-prepared, (b) 400, (c) 500 and (d) 600ºC for 1 h |
3.3. Sensing Response Properties
- The sensing property of metal oxide-based sensors under ethanol is investigated by allowing ethanol vapor in dry air. The concentration of ethanol vapor in dry air is calculated using the ideal gas equation at room temperature and at atmospheric pressure. According to gas equation PV = NkT (at STP) where, ‘P’ is the standard pressure (760 mm of Hg), ‘V’ is the volume of the chamber (25000 cc) and ‘k’ is the Boltzmann constant. A known concentration (100 ppm) of ethanol gas was controlled by the injection using a micro-syrings inserted in the vacuum chamber. The injected solvents were completely vaporized on a hot glass plate (about 75ºC). The injection volume (Vi) was calculated for the evaluation of gas concentration (c in ppm) by:
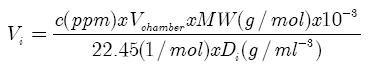 | (2) |
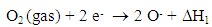 | (3) |
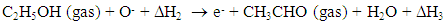 | (4) |
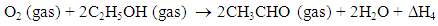 | (5) |
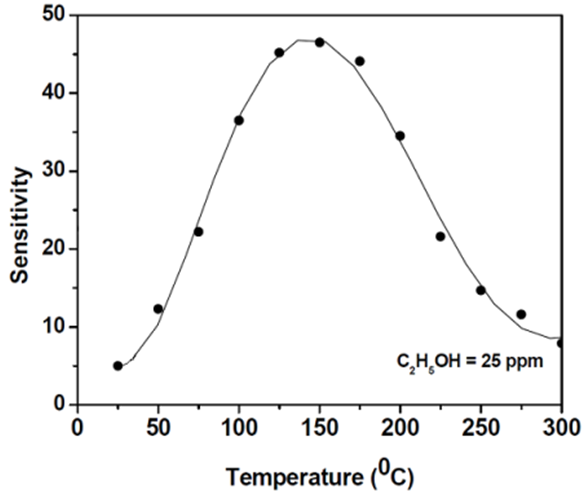 | Figure 6. Response curve to 25 ppm of C2H5OH gas at different operation temperatures |
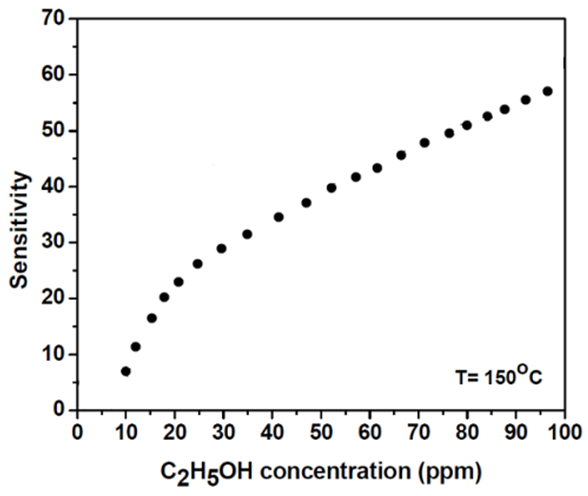 | Figure 7. Response curve to different concentrations of C2H5OH gas at the optimal temperature (T = 150°C) |
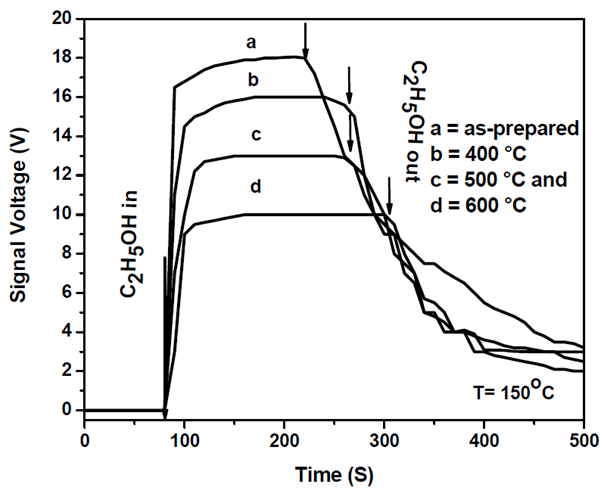 | Figure 8. The as-prepared and different calcination temperature ZnO based thick-film sensor samples response curves for 25 ppm of C2H5OH gas at optimal temperature (T = 150°C) |
4. Conclusions
- The morphology of ZnO nanorods were controlled during the microwave-assisted combustion synthesis method and their C2H5OH sensing characteristics were investigated. Well dispersed ZnO nanorods could be prepared by adding a small amount of water to the solution containing Zn(NO3)2 · 6H2O, and glycine as fuel. The morphological change in the ZnO nanorods was attributed to the various calcination temperatures. At the calcination temperature of 400 to 600°C, the well-dispersed ZnO nanorods are observed. The present ZnO nanorods are suitable for use in air quality sensor is as a breath analyzer, to detect C2H5OH in the presence of ethanol vapor in human breath is correlated with the concentration in the blood. Therefore, microwave-assisted combustion synthesis method prepared ZnO have been shown to demonstrate excellent sensing properties.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML