Tei-Chen Chen, Ruei-Huan Lin
Department of Mechanical Engineering, National Cheng Kung University, Tainan, Taiwan
Correspondence to: Tei-Chen Chen, Department of Mechanical Engineering, National Cheng Kung University, Tainan, Taiwan.
| Email: |  |
Copyright © 2015 Scientific & Academic Publishing. All Rights Reserved.
Abstract
In the recent years, the LiFePO4 materials with the olivine structure have become a promising cathode material for the lithium ion battery. LiFePO4 has a lot of advantage, such as high operation voltage, long operational life, low materials cost, environmental friendliness. However, the disadvantages of low electronic conductivity and poor ionic conductivity greatly restrict the commercial applications of LiFePO4. Metal doping is one of the effective ways to improve materials properties of the LiFePO4. In this study, the materials properties of LiFePO4 after doping metal ions were performed by first-principles calculation. It was found that doping metal atoms to LiFePO4 can significantly reduce the volume variation during the lithiation/ delithiation cycles. Consequently, the working life of cathode materials can be improved. The diffusion of Li-ion is dependent on the hopping distance. The metal doping in LiFePO4 leads to the increase of hopping distance. This expansion effect would benefit the Li ion diffusion. The effects of metal doping on the electronic structures were performed by the investigation of band structure. The results show that doping metal ion into LiFePO4 induces a narrowing of the band gap, which could benefit to improve the electronic conductivity. From the analysis of the density of states, it was found that the energy bands near the Fermi energy were mainly attributed to the doping metal atoms. This result leads to the decrease of energy gap between the valence band and conduction band. In this work, V-ion doping shows an optimum effect than other elements under consideration. The band gap of V-ion doping (0.2068eV) is much smaller than the band gap of un-doped LiFePO4 (0.9245eV).
Keywords:
Li-Ion batteries, Metal doping, LiFePO4, Band gap, First-Principles calculation
Cite this paper: Tei-Chen Chen, Ruei-Huan Lin, Effects of Metal Doping on Properties of LiFePO4 Cathode Material by First-Principle Calculation, International Journal of Materials Engineering , Vol. 5 No. 5, 2015, pp. 121-124. doi: 10.5923/j.ijme.20150505.03.
1. Introduction
In the recent years, the LiFePO4 materials with the olivine structure have become a promising cathode material for the lithium ion battery. LiFePO4 has a lot of advantages, such as high operation voltage, long operational life, low materials cost, environmental friendliness. However, the disadvantages of low electronic conductivity and poor ionic conductivity greatly restrict the commercial applications of LiFePO4. Metal doping is one of the effective ways to improve materials properties of the LiFePO4 [1-2]. In this study, the materials properties of LiFePO4 by doping metal ions were investigated by first-principles calculation and some findings were illustrated.
2. Theoretical Approach
The calculations are carried out to investigate the geometry structures and electronic properties of LiFePO4 material using Vienna Ab initio Simulation Package (VASP) based on density functional theory (DFT). The generalized gradient approximation (GGA) within Perdew-Wang 91 functional is employed to take account of the electronic exchange-correlation effect. The cutoff energy of 400 eV is used in calculation. LiFePO4 is an olivine structure. The unit cell with lattice parameters and volume a=10.3324(Å), b=6.0105 (Å), c=4.6922(Å), and V=263.77 (Å3), respectively, are constructed. The structure of LiFePO4 material and the minimum energy path of Li-ion diffusion are as shown in Figs. 1 and 2, respectively. The unit cell of LiFePO4 and the model to depict four Li ions delithiated away individually from the unit cell of LiFePO4 are shown in Figs. 3 and 4, respectively.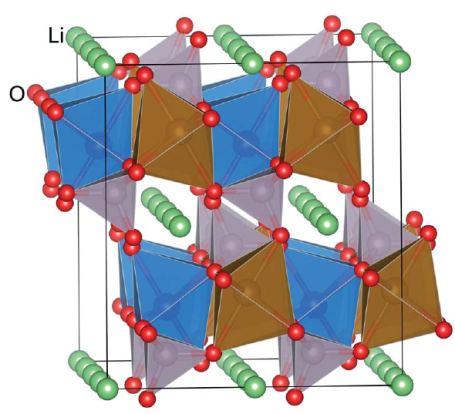 | Figure 1. Structure of LiFePO4 material [3] |
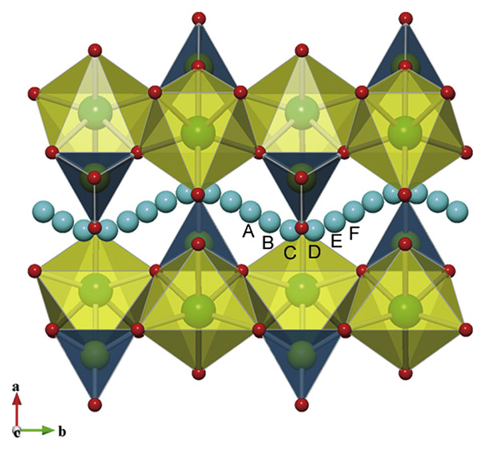 | Figure 2. Minimum energy path of Li-ion diffusion [3] |
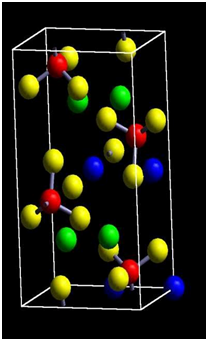 | Figure 3. Unit cell of LiFePO4 |
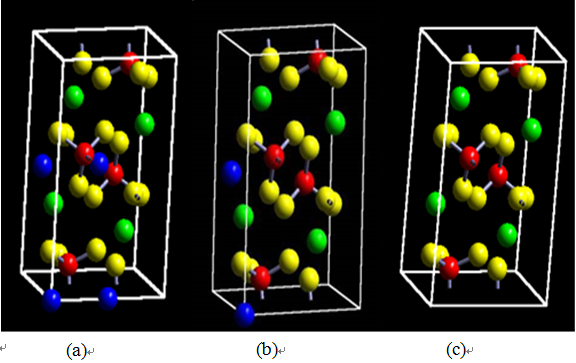 | Figure 4. Schematic diagram of Li ion delithiation (a) Li compound phase (b) intermediate phase (c) delithiation phase |
3. Results and Discussion
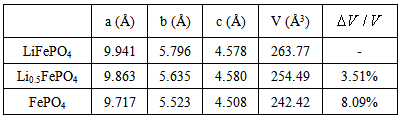
The changes of lattice parameters and volume after discharge of Li ions are shown in Table 1. It is seen that the lattice parameters tend to decrease and the volume change of cell shrinks as large as 8.09% when the Li ions move away from the lattice.Table 1. Lattice parameters and volume change of LiFePO4 after discharge
 |
| |
|
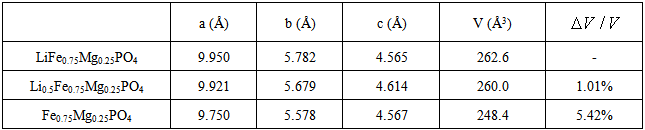
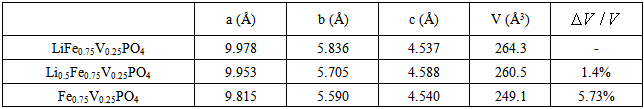
During the charging process of Li-ion battery, the extraction of Li ions will induce a significant volume change of cathode material. It is found that the change of bond length of P-O is small, but it is quite large for Fe-O after extraction of Li-ion in LiFePO4. In other words, the volume change of LiFePO4 material is mainly due to the change of bond length of Fe-O rather than P-O after discharge. In this work, effects of doping Mg, V and Co metal ions on volume change are evaluated and as shown in Tables 2, and 3, respectively. Compared to the significant change in bonding length of Fe-O, the change in bonding length M-O of doping metals M (M=Mg, V, Co) in doping LiFePO4 material is much smaller after discharge. These stable structures have a smaller change in volume after discharge.Table 2. Lattice parameters and volume change of LiFe0.75Mg0.25PO4
 |
| |
|
Table 3. Lattice parameters and volume change of LiFe0.75V0.25PO4
 |
| |
|
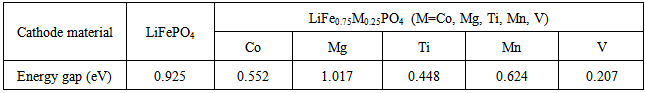
High performance of Li-ion battery should have high diffusivity in cathode material. However, the diffusivity of LiFePO4 cathode material is only about 10-11~10-13 cm2s-1, much lower than the 10-8 cm2s-1 of LiCoO2, already popularly used in market. In general, the diffusive behavior of Li-ion is related to the gap of lattice structure, which is the main pathway of Li-ions. Larger gap allows more Li-ion passing through. For instance, LiCoO2 is characterized by two-dimensional layer structure and the gap between two adjacent layers is large enough and can accommodate a lot of Li-ions to pass through. It, therefore, has a very good diffusivity. On the other hand, LiFePO4 is featured by P-O tetrahedron and Fe-O octahedral structures, and the pathway of Li-ion is only one-dimensional, which severely restricts the diffusivity of material. In this article, a part of Li atoms in LiFePO4 are replaced by Na through doping.The electronic conductivity of LiFePO4 material is limited due to large energy gap. It was reported in experiment that the V-doped LiFePO4 cathode exhibits significant enhancement in the electrochemical performance compared with pure olivine LiFePO4 [4]. In this work, the effects of doping five different elements, i.e., Mg, V, Ti, Mn, and Co, to LiFePO4 material are studied. It is found in Table 4 that the energy gap can be significantly reduced by doping V, Ti, Mn, and Co. The effect of doping V is the best of all. Compared to Figs. 3 and 4, the decrease of energy gap by doping V is mainly due to the shift of density state toward the Fermi energy in conduction band, as shown in Fig. 5.Table 4. Energy gap of cathode materials after doping M (M = Co, Mg, Ti, Mn, V)
 |
| |
|
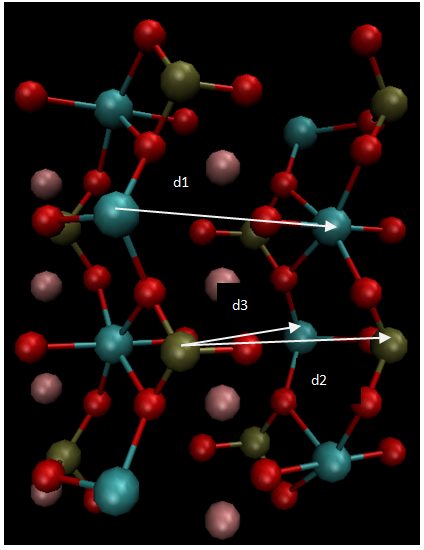 | Figure 2. Molecular structure of LiFePO4 |
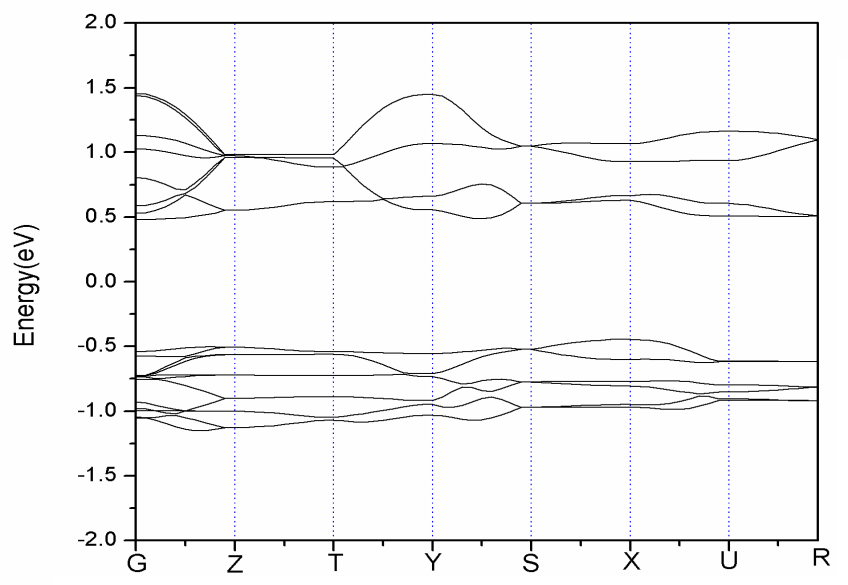 | Figure 3. Energy band structure of LiFePO4 |
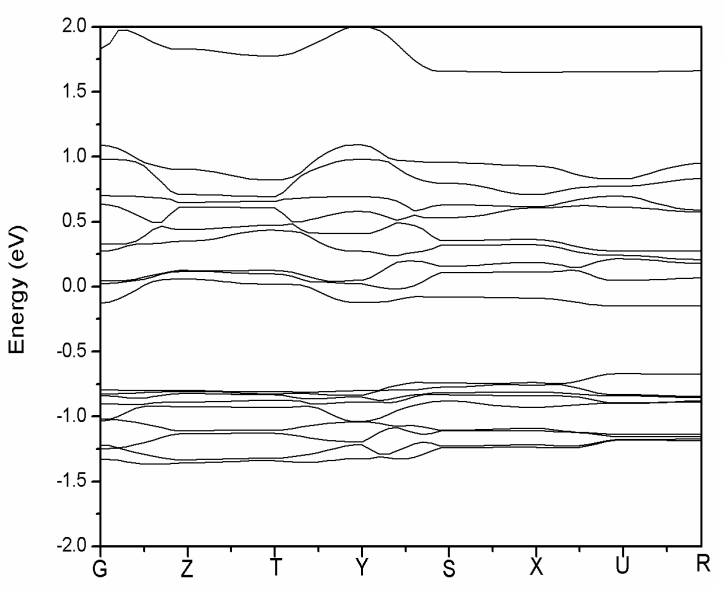 | Figure 4. Energy band structure of Li0.75Na0.25Fe0.75V0.25PO4 |
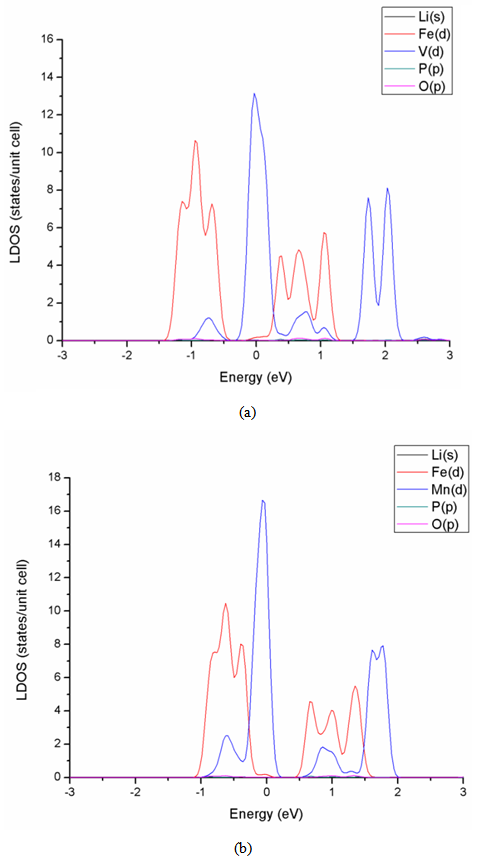 | Figure 5. LDOS of metal doping (a) LiFe0.75V0.25PO4 (b) LiFe0.75Mn0.25PO4 |
4. Conclusions
1. In this work, the effects of doping Mg, V, Ti, Mn, and Co to LiFePO4 material by replacing Fe atoms to improve the electric conductivity were evaluated. It was found that the energy gap can be reduced by doping V, Ti, Mn, and Co. The effect of doping V is the optimal, and the corresponding energy gap can be significantly reduced from 0.925 eV to 0.207 eV. However, the effect of doping Mn is insignificant. The reason why the energy gap becomes smaller is mainly due to the shift of local electron distributions toward the Fermi energy. 2. During the charge/discharge cycles, the volume variation of LiFePO4 material becomes significant due to the repeatedly extraction/insertion of Li ions. Higher volume change tends to cause higher possibility of structural damage. It was found that the doping of Mg, V, and Co metal ions can decrease the volume change and improve the life cycles of battery. For these three metals, the effect of Mg is the best. The volume change can be significantly reduced from original 8.1% to 5.4%.3. The diffusion efficiency of Li ion in LiFePO4 material can be enhanced by increasing the gap of LiFePO4. It was found that the gap can be increased by doping Na ion.
ACKNOWLEDGEMENTS
This research was supported by the Ministry of Science and Technology in Taiwan through Grants No. 103-2221-E-006-054.
References
| [1] | S. L. Shang, Y. Wang, Z. G. Mei, X. D. Hui, and Z. K. Liu, "Lattice dynamics, thermodynamics, and bonding strength of lithium-ion battery materials LiMPO4 (M = Mn, Fe, Co, and Ni): a comparative first-principles study," Journal of Materials Chemistry, vol. 22, pp. 1142-1149, 2012. |
| [2] | H. Lin, Y. W. Wen, C. X. Zhang, L. L. Zhang, Y. H. Huang, B. Shan, and R. Chen, "A GGA+U study of lithium diffusion in vanadium doped LiFePO4," Solid State Communications, vol. 152, pp. 999-1003, Jun 2012. |
| [3] | M. S. Islam, D. J. Driscoll, C. A. J. Fisher, and P. R. Slater, “Atomic-scale investigation od defects, dopants, and lithium transport in the LiFePO4 olivine-type battery material, “ Chemistry of Materials, vol. 17, pp.5082-5092, 2005. |
| [4] | S. Franger, F. Le Cras, C. Bourbon, H. Rouault, "LiFePO4 synthesis routes for enhanced electrochemical performance," Electrochemal and Solid-State Letters, vol. 5, A231-A233, 2002. |








 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML


