-
Paper Information
- Next Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
International Journal of Materials Engineering
p-ISSN: 2166-5389 e-ISSN: 2166-5400
2014; 4(5): 171-179
doi:10.5923/j.ijme.20140405.02
Inhibition of Mild Steel Corrosion in HCl Solution by Pentaclethra macrophylla Bentham Extract
Lebe A. Nnanna1, 2, Israel O. Owate2, Emeka E. Oguzie3
1Materials Science group, Physics/Electronics Department, Abia State Polytechnic, Aba, Nigeria
2Physics Department, University of Port Harcourt, Port Harcourt, Nigeria
3Electrochemistry Lab, Federal University of Technology, Owerri, Nigeria
Correspondence to: Lebe A. Nnanna, Materials Science group, Physics/Electronics Department, Abia State Polytechnic, Aba, Nigeria.
| Email: |  |
Copyright © 2014 Scientific & Academic Publishing. All Rights Reserved.
Weight loss and electrochemical techniques (open circuit potential, linear sweep voltammetry and potentiodynamic polarization) were used to assess the effectiveness of Pentaclethra macrophylla Bentham (PMB) extracts as corrosion inhibitor for mild steel in 1.0 M HCl solution at 30 - 45℃. It was found that Pentaclethra macrophylla Bentham extracts retarded the dissolution of mild steel in 1.0 M HCl solution. The inhibition efficiency increased with increase in extract concentration and did significantly well at increased temperature, which is suggestive of physical and chemical adsorption mechanism. Open circuit potential shows reduction of resistance polarization Rp with the addition of the PMB extract. Potentiodynamic polarization result suggests that PMB extracts functioned as mixed-type inhibitor. The adsorption of PMB extracts onto the mild steel surface followed Langmuir and Temkin adsorption isotherm models. The mechanism of physisorption of the extracts onto the mild steel surface is proposed from the trend of inhibition efficiency with temperature which is corroborated by the values of activation parameters obtained from the experimental data.
Keywords: Acid corrosion, Mild steel, Corrosion inhibition, Adsorption, Pentaclethra macrophylla Bentham
Cite this paper: Lebe A. Nnanna, Israel O. Owate, Emeka E. Oguzie, Inhibition of Mild Steel Corrosion in HCl Solution by Pentaclethra macrophylla Bentham Extract, International Journal of Materials Engineering , Vol. 4 No. 5, 2014, pp. 171-179. doi: 10.5923/j.ijme.20140405.02.
Article Outline
1. Introduction
- Corrosion inhibitors are one of the practical and cost effective methods of controlling metallic corrosion. They may be inorganic or organic substances. Inorganic substances suitable as metal corrosion inhibitor must easily oxidize the metal to form an impervious layer which prevent direct ions-metal interaction and hence retard the rate of metal dissolution in the medium. The organic counterpart, on the other hand, must possess features including presence of heteroatoms and/or double bonds, large surface area, active centre, etc. which upon adsorption on the metal surface will blanket a large area of the metal and thus isolate it from the aggressive ions present in the environment. The use of inorganic (silicates, nitrates, nitrites, molybates, phosphates, and borates) and organic (predominantly, those with O, N, S, and P heteroatoms) compounds have been reported in the literature as metal corrosion inhibitors in different corrosive environments (Fouda et al., 2006; Oguzie et al., 2007; Achary et al., 2008; Ashassi and Ghalebsaz, 2005; Chidiebere et al., 2012). However, the toxic nature of most inorganic metal corrosion inhibitors and the exorbitant prices of the organic counterparts are the major setbacks for their continuous use. The search for an efficient inhibitor for metal inhibitor for metal corrosion in different aggressive media has, in recent times, taken a new dimension owing to the clarion call for green chemistry. Moreover, since the whole idea of metal protection is anchored on economic gain and environmental sustainability, the substance to be used as metal corrosion inhibitor must be cheap, readily available, and environmentally friendly. Hence, research activities are geared towards finding a replacement for inorganic and organic metal corrosion inhibitors. Plant is one of the sources of cheap, readily available, and non-toxic green metal corrosion inhibitors. Plant products are organic in nature, and contain certain photochemical including tannins, flavonoids, saponins, organic and amino acids, alkaloids, and pigments which could be extracted by simple less expensive procedures. Extracts from different parts of plant have been widely reported as effective and good metal corrosion inhibitors in various corrosive environments (Nnanna et al., 2014; Gerengi and Sahin, 2012; Ji et al., 2011; Krishnegowda et al., 2013; Umoren et al., 2013; Okafor et al., 2008). To enhance the efficiency of metal corrosion inhibitors, extensive studies have been undertaken to identify the synergistic effect of other additives. Li et al., (2008) noted that synergism provides a way of improving the inhibitive force of inhibitor, decreasing the quantity of inhibitor usage, and diversifying the application of the inhibitor in an aggressive environment. The present work reports on the inhibitive effect of Pentaclethra macrophylla Bentham root extracts for mild steel in hydrochloric acid solution using chemical and electrochemical techniques in furtherance of our continuous quest to explore green corrosion inhibitors.
2. Experimental
2.1. Materials Preparation
- A flat sheet of mild steel 0.05 cm in thickness with the following composition: C = 0.05%; Mn = 1.13%; Si = 0.05%; P = 0.91%; S = 0.85%; Cu = 0.09%; Pb = 0.15%; Ve 0.13%; Mo = 0.08%; and the balance Fe was used in the study. The mild steel was mechanically press-cut into coupons of 2 cm × 2 cm dimensions for weight loss measurements and 1 cm × 1 cm for electrochemical measurements. The coupons were ground with different grades (# 400, 600, 800 and 1000) silicon carbide paper, degreased in absolute ethanol, dried in warm air and stored in moisture-free desiccators prior to use. The aggressive medium was 1 M HCl prepared from 98% analytical grade supplied by Sigma-Aldrich. Deionised water was used for the preparation of all reagents. The Pentaclethra macrophylla Bentham root was obtained from the suburb of Owerri, Nigeria, air dried and ground to fine powder. 10 g of the ground roots was extracted using reflux apparatus for 3 h. The extracts were made into various concentrations.
2.2. Weight Loss Measurements
- Weight loss measurements were performed using 250 ml capacity beakers containing 200 mL test solution under total immersion in stagnant aerated condition at 30-45℃ maintained in a thermostated water bath. The pre-cleaned and weighed mild steel coupons were suspended in beakers with the help of rods and hooks. The coupons were withdrawn at 2 h interval progressively for 24 h, cleaned as previously reported and reweighed. The weight loss in grams was taken as the difference in the weight of the mild steel coupons before and after immersion in different test solutions. Tests were performed for the blank solution (1 M HCl), Pentaclethra macrophylla Bentham root extracts concentrations of 0.1 – 0.5 g/l at different temperatures. The experiments were done in triplicate to ensure reproducibility. The standard deviation values among parallel triplicate experiments were found to be less than 5%, indicating good reproducibility. From the weight loss values, corrosion rates were computed using the expression:
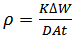 | (1) |
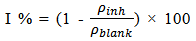 | (2) |
2.3. Electrochemical Measurements
- The electrochemical experiments were performed using a VERSASTAT 400 complete dc voltammetry and corrosion system, with V3 Studio software. A conventional three-electrode Pyrex glass cell was used for the experiments. Test coupons with 1 cm2 exposed areas were used as working electrode and a graphite rod as counter electrode. The reference electrode was a saturated calomel electrode (SCE), which was connected via a Luggin’s capillary. The test electrolyte was 1 M solution of HCl. All experiments were undertaken in stagnant aerated solutions at 30 ± 1℃. The working electrode was immersed in attest solution for one hour until a stable open circuit potential was attained. The linear sweep voltammetry (LSV) tests were made at corrosion potential (Ecorr) over a frequency range of 100 kHz to 100 MHz, with a signal amplitude perturbation of 2 mV. The potentiodynamic polarization study was from cathodic potential of -250 mV to anodic potential of +250 mV with respect to the corrosion potential at a sweep rate of 1 mV/s. The linear Tafel segments of the anodic and cathodic curves were extrapolated to corrosion potential to obtain the corrosion current densities (icorr). Each experiment was carried out three times to estimate reproducibility and average values of the electrochemical parameters are reported.
2.4. EDS and SEM Surface Morphology
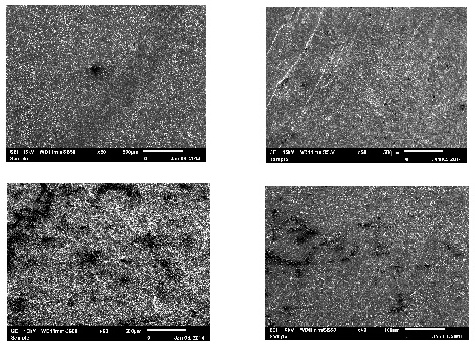
- The analysis of the morphology of the mild steel surface was carried out using scanning electron microscopy (SEM), operated in the contact mode under ambient conditions using JSM-6010LA analytical scanning electron microscope (JOEL Technologies, USA). Images of the specimens were recorded after 12 h exposure time in 1 M HCl without and with various concentrations of the extract inhibitor. The EDS of the extract inhibitor was performed so as to ascertain the compound composition. The result of the EDS micrograph confirmed the presence of the heteroatoms of oxygen and phosphate which authenticates the root extract to be a good and efficient inhibitor.
3. Results and Discussion
3.1. Preliminary Phytochemical Screening
- The phytochemical analysis of the leaves and seed extracts of Pentaclethra macrophylla Bentham has the followings; tannins, saponins, flavonoids, alkaloids and phenols (Dongmo et al., 2007; Oyeleke et al., 2014). The presence of these active phytoconstituents in the extracts may have great influence on their corrosion inhibition property. This suggests that the root extracts Pentaclethra macrophylla Bentham (Ugba root) is a good and efficient inhibitor of corrosion of mild steel within the environment.
3.2. Weight Loss Measurements
- The assessment of the effectiveness of a metal corrosion inhibitor were done using chemical and electrochemical methods. For the chemical techniques, weight loss measurements have been extensively employed for long-term immersion test. Corroborative results between weight loss and other corrosion monitoring techniques have been reported by some researchers (Umoren et al., 2012; Mousa et al., 1998; Ebenso & Oguzie, 2005). The variation of weight loss against time for mild steel corrosion in 1 M HCl without and with different concentrations of Pentaclethra macrophylla Bentham root extracts at different temperatures (30-45℃). Fig. 2 shows the plot of corrosion rate versus extract concentration for Pentaclethra macrophylla Bentham root extracts. Inspection of the figures revealed that the corrosion rate of mild steel in 1 M HCl solution was reduced upon the introduction of Pentaclethra macrophylla Bentham root extracts into the corrosive medium. The extent of reduction in corrosion rate is seen to increase with increase in the concentration of the Pentaclethra macrophylla Bentham root extracts. The plot of the inhibition efficiency against extract concentration from weight loss experiments is shown in Fig. 3. Close examination of Fig. 3 showed that the root extracts of PMB afforded protection for mild steel in 1 M HCl solution which could be attributed to the adsorption of the components of the extracts on the mild steel surface. The figure further revealed that the inhibition efficiency increased with increase in the concentration of Pentaclethra macrophylla Bentham root extracts but decreased slightly as the temperature was raised. The decrease in the inhibition efficiency of Pentaclethra macrophylla Bentham root extracts as the temperature increases may be attributed to the desorption of the adsorbed components of the extracts on the mild steel surface. It is therefore pertinent to conclude that the high inhibition efficiency of the extracts observed in the present study could be attributed to high phytochemical constituents of tannins, flavonoids and saponins. Also, the presence of heteroatoms (O, P) as revealed by the EDS micrograph serve as the adsorption sites of the extracts onto the mild steel surface and thus enhances protection of the mild steel from corrosive agents present in the aqueous medium.
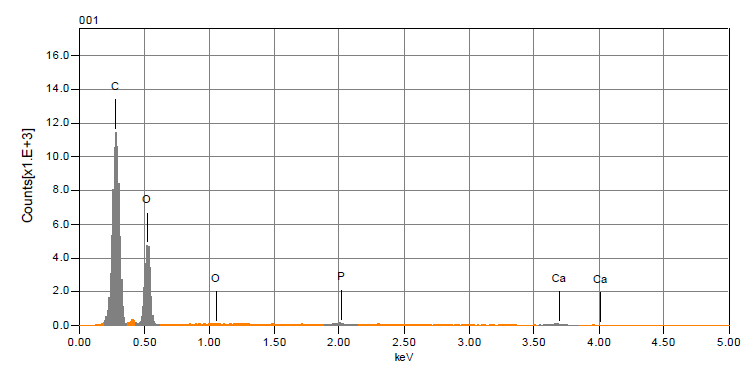 | Figure 1. EDS Micrograph of Ground Pentaclethra macrophylla Bentham Roots |
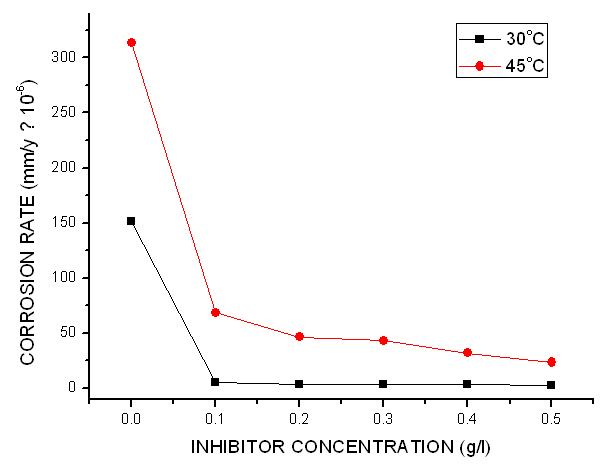 | Figure 2. Corrosion rate of the mild steel in 1.0 M HCl with and without the inhibitor |
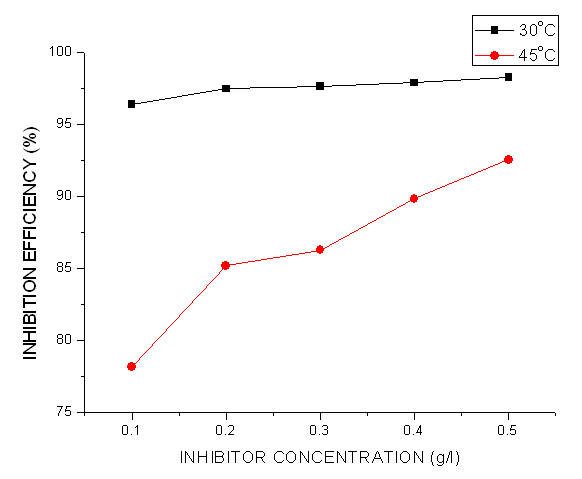 | Figure 3. Inhibition efficiency of the Pentaclethra macrophylla B. in the corrosion of mild steel in 1.0 M HCl |
3.3. Adsorption Considerations
- Adsorption at the metal-solution interface has been considered as the primary step in the action of most organic corrosion inhibitors in acidic environment. Langmuir adsorption isotherm was used to deduce whether there is formation of layer of insoluble complex of the metal on the surface which acts as a barrier between the metal surface and the corrosive medium - usually termed physisorption, while Temkin isotherm was used to determine if the extract adsorption on the metal surface is via chemisorption which involves displacement of water molecules from the metal surface and the sharing of electrons between oxygen atom and iron. Langmuir plot describes the relationship between the surface coverage and inhibition concentration of a material as:
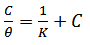 | (3) |
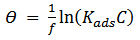 | (4) |
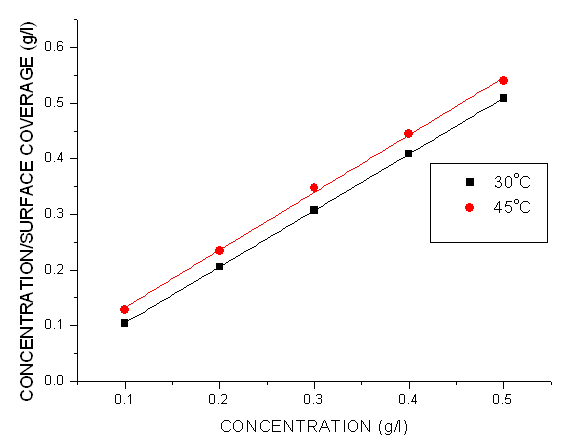 | Figure 4. Langmuir Isotherm Plots of Corrosion of Mild Steel in 1.0 M HCl in the presence of Pentaclethra macrophylla Bentham Root Extract |
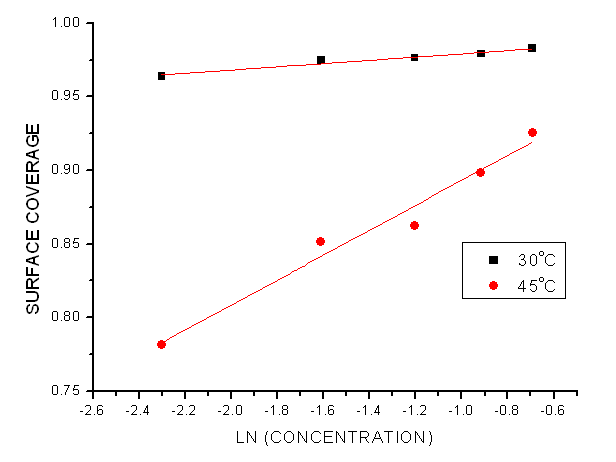 | Figure 5. Temkin Isotherm Plots of the Corrosion of Mild Steel in 1.0 M HCl in the presence of Pentaclethra macrophylla Bentham Root Extract |
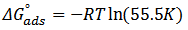 | (5) |
3.4. Effect of Temperature
- The influence of temperature on the corrosion behaviour of mild steel in the absence and presence of Pentaclethra macrophylla Bentham root extracts in 1 M HCl solution was investigated at temperature range of 30-45℃. Analysis of the temperature dependence of inhibition efficiency as well as comparison of corrosion apparent activation energies in absence and presence of inhibitor gives some insight into the possible mechanism of inhibitor adsorption. A decrease in inhibition efficiency with the rise in temperature with analogous increase in corrosion activation energy in the presence of inhibitor compared to its absence is frequently interpreted as being suggestive of formation of an adsorption film of physical (electrostatic) nature. The reverse effect, corresponding to an increase in inhibition efficiency with rise in temperature and lower activation energy in the presence of inhibitor, suggests a chemisorptions mechanism (Ashassi-sorkhabi et al., 2006, Oguzie et al., 2004, Ebenso, 2003).
3.5. Electrochemical Measurements
3.5.1. Open Circuit Potential
- The variation of the open circuit potential with time for mild steel corrosion in 1 M HCl solution without and with selected concentrations of Pentaclethra macrophylla Bentham root extracts is shown in Fig. 4. It is clear from the curves that the corrosion potential of the blank solution increased steadily and reached a maximum value of -0.42064 V. However, 0.3 g/l concentration of the extract had the best curve when compared to 0.1 g/l and 0.5 g/l.
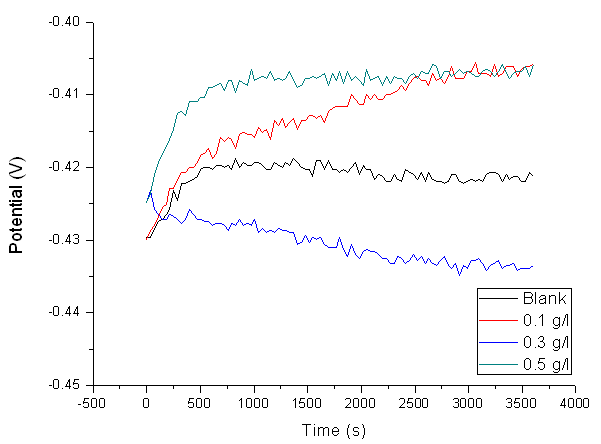 | Figure 6. OCP Curve of the Corroded Mild Steel in 1.0 M HCl in the absence and Different Concentration of Pentaclethra macrophylla Bentham Root Extract |
3.5.2. Potentiodynamic Polarization Measurements
- Potentiodynamic polarization measurements were undertaken mainly to gain an insight into the possible influence of addition of different concentrations of Pentaclethra macrophylla Bentham root extracts on the anodic dissolution of mild steel and the corresponding cathodic reduction of hydrogen ion of the corrosion process. Typical anodic and cathodic polarization curves obtained for the corrosion of mild steel in 1 M HCl solution in the absence and presence of selected concentrations of Pentaclethra macrophylla Bentham root extracts are presented in Fig. 5. The curves showed that the introduction of the additives to the blank solution has modest influence on both anodic and cathodic half reactions, although cathodic influence appeared much pronounced. The potentiodynamic parameters obtained by extrapolation of the linear Tafel segments of the anodic and cathodic curves are presented in Table 1. The results in the table indicate that the introduction of the various concentrations of Pentaclethra macrophylla Bentham root extracts remarkably shift Ecorr /SCE. For instance, the difference between the Ecorr /SCE of the blank solution and that of the highest concentration of PMB extract is 33.8 mV. It could be inferred that the extracts acted as a mixed-type inhibitor and the inhibition is due to simple geometric blocking mechanism (Eduok et al., 2012). Also, the corrosion current densities of the additives decreased significantly compared to the blank. It is clear also from the result (Table 1) that the βa and βc values for the various concentrations of the extracts reduced remarkably compared to the blank indicating that the additive simultaneously modified both the anodic and cathodic reactions thus supporting the assertion that the extract is a mixed-type inhibitor. The corrosion inhibition efficiency of Pentaclethra macrophylla Bentham root extracts was calculated using the relation:
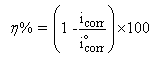 | (6) |
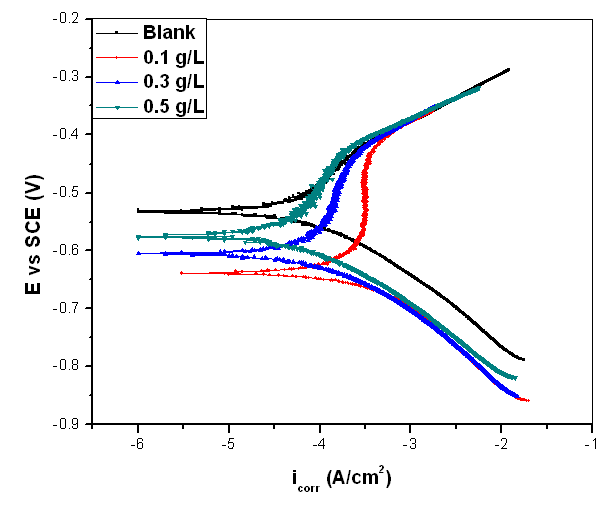 | Figure 7. Potentiodynamic polarization curves of mild steel in 1.0 M HCl without and with different concentrations of Pentaclethra macrophylla Bentham Roots Extract |
3.5.3. Micrograph of the Corroded Metals
- The SEM image clearly shows that the corrosion reaction does not take place homogenously over the surface of mild steel in 1.0 M HCl solution. However, the surface is remarkably protected by Pentaclethra macrophylla Bentham root extracts, in comparison to the inhibitor-free solution.
4. Conclusions
- The following conclusions can be drawn from the work.1. Pentaclethra macrophylla Bentham root extracts act as an inhibitor for mild steel corrosion in 1 M HCl solution. Inhibition efficiency increased with increase in the concentration of the inhibitor.2. Potentiodynamic polarization results indicate that Pentaclethra macrophylla Bentham root extracts behave as mixed-type inhibitor.3. Corrosion inhibition is afforded by both physical and chemical adsorption components of Pentaclethra macrophylla Bentham root extracts on the mild steel surface. Therefore, Langmuir and Temkin adsorption isotherms are obeyed.4. Results from all the techniques employed are in reasonably good agreements and points to the fact that Pentaclethra macrophylla Bentham root extracts is a good and efficient inhibitor within the range of concentrations investigated.
ACKNOWLEDGEMENTS
- We acknowledge the financial support from Tertiary Education Trust Fund (TETFUND) of Nigeria. We are also grateful to Purdue Water Institute, Calumet, Purdue University, Calumet, Indiana, USA for the technical assistance in performing electrochemical measurements, EDS and SEM surface morphology.
References
| [1] | A.S. Fouda, A.A. Al-Sarawy, E.E. El-Katori, Pyrazolone derivatives as corrosion inhibitors for C-steel in hydrochloric acid solution, Desalination 201 (2006) 1–3. |
| [2] | E.E. Oguzie, Y. Li, F.H. Wang, Effect of 2-amino-3-mercaptopropanoic acid (cysteine) on the corrosion behaviour of low carbon steel in sulphuric acid, Electrochimica Acta 53 (2007) 909–14. |
| [3] | G. Achary, H.P. Sachin, Y.N. Arthola, T.V. Venkatesha, The corrosion inhibition of mild steel by 3-formyl-8-hydroxy quinoline in hydrochloric acid medium, Materials Chemistry and Physics 107 (2008) 44–50. |
| [4] | H. Ashassi-Sorkhabi, N. Ghalebsaz-Jeddi, Inhibition effect of polyethylene glycol on the corrosion of carbon steel in sulphuric acid, Materials Chemistry and Physics 92 (2005) 480–6. |
| [5] | M.A. Chidiebere, C.E. Ogukwe, K.L. Oguzie, C.N. Eneh, E.E. Oguzie, Corrosion inhibition and adsorption behavior of Punica granatum extract on mild steel in acidic environments: experimental and theoretical studies, Industrial & Engineering Chemistry Research 51 (2012) 668 – 77. |
| [6] | L.A. Nnanna, I. O. Owate, O.C. Nwadiuko, I.I. Dike, J.O. Isu (2014). Inhibition of mild steel corrosion by Aspilia africana in acidic solution. American J. Mat. Sci. 4(3): 144 – 149. |
| [7] | H. Gerengi, H.I. Sahin, Schinopsis lorentzii extract as a green corrosion inhibitor for low carbon steel in 1 M HCl solution, Industrial & Engineering Chemistry Research 51 (2012) 780–7. |
| [8] | G. Ji, S.K. Shukla, P. Dwivedi, S. Sundaram, R. Prakash, Inhibitive effect of Argemone mexicana plant extract on acid corrosion of mild steel, Industrial & Engineering Chemistry Research 50 (2011) 11954–9. |
| [9] | P.M. Krishnegowda, V.T. Venkatesha, P.K.M. Krishnegowda, S.B. Shivayogiraju, Acalypha torta leaf extract as green corrosion inhibitor for mild steel in hy- drochloric acid solution, Industrial & Engineering Chemistry Research 52 (2013) 722–8. |
| [10] | S.A. Umoren, Z.M. Gasem, I.B. Obot, Natural products for materials protection: inhibition of mild steel corrosion by date palm seed extracts in acid media, Industrial & Engineering Chemistry Research 52 (2013) 14855–65. |
| [11] | P.C. Okafor, M.E. Ikpi, I.E. Uwah, E.E. Ebenso, U.J. Ekpe, S.A. Umoren, Inhibitory action of Phyllanthus amarus extracts on the corrosion of mild steel in acidic media, Corrosion Science 50 (2008) 2310–17. |
| [12] | X. Li, S. Deng, H. Fu, G. Mu, Synergism between rare earth cerium(IV) in and vanillin on the corrosion of cold rolled steel in 1 M HCl solution, Corrosion Science 50 (2008) 3599–609. |
| [13] | G.O. Oyeleke, J.O. Odedeji, A.D. Ishola, O.Afolabi. Phytochemical Screening and Nutritional Evaluation of African Oil Bean (Pentaclethra macrophylla) Seeds IOSR Journal of Environmental Science, Toxicology and Food Technology, 8(2014), 14-17. |
| [14] | F.G. Dongmo, O.J. Enyong, M.C. Noumessing, M.D. Enyegue (2007). Phytochemical Constituents and Antioxidant Potential of some Cameroonian Medicinal Plants. Pharmacologyonline 2(2007) 436-452. |
| [15] | S.A. Umoren, U.M. Eduok, A.U. Israel, I.B. Obot, M.M. Solomon, Coconut coir dust extract: a novel eco-friendly corrosion inhibition for Al in HCl solutions, Green Chemistry Letters and Reviews 5 (2012) 303–9. |
| [16] | M.N. Moussa, A.S. Foula, A.I. Taha, A. Elnenaa, Some thiosemicarbazide derivatives as corrosion inhibitors for aluminium in sodium hydrogen solution, Bulletin of the Korean Chemical Society 9 (1998) 192–5 . |
| [17] | E.E. Ebenso, E.E. Oguzie, Corrosion inhibition of mild steel in acidic media by some organic dyes, Materials Letters 59 (2005) 2163–5. |
| [18] | A. Popova, E. Sokolova, S. Raicheva, M. Christov (2003), AC and DC study of the temperature effect on mild steel corrosion in acid media in the presence of benzimidazole derivatives, Corrosion Science, 45(1):33–58. |
| [19] | S. Rajendran, M. R. Joany, B. V. Apparao, N. Palaniswamy (2000), Synergistic effect of calcium gluconate and Zn2 on the inhibition of corrosion of mild steel in neutral aqueous environment, Trans. SAEST. 35(3, 4):113. |
| [20] | E. Oguzie (2005), Inhibition of acid corrosion of mild steel by Telfaria occidentalis extract, Pigment Resin Technol., 34(6):321-326. |
| [21] | S. T. Arab, A. M. Turkustuni (2006), Inhibition of the corrosion of steel in phosphoric acid by phenacyl dimethyl sulfonium bromide and some of its parasubstituted derivatives, Portugalia. Electrochim. Acta. 24:53-69. |
| [22] | H. Ashassi-sorkhabi B. Shaabani, B. Aligholipour, D. Seifzadeh (2006), The effect of some Schiff bases on the corrosion of aluminium in HCl solution, Appl. Surf. Sci., 252:4039-4047. |
| [23] | E. Oguzie, G. Onuoha, A. Onuchukwu (2004), Influence of halide ions on inhibitive effect of congo red dye on the corrosion of mild steel in sulphuric acid solution, Mater. Chem. Phys. 89: 305. |
| [24] | E. Ebenso (2003), Effect of Halide ions on the corrosion inhibition of mild steel in H2SO4 using methyl red. Part 1, Bull Electrochem.19, 209-216. |
| [25] | U.M. Eduok, S.A. Umoren, A.P. Udoh, Synergistic inhibition effects between leaves and stem extracts of Sida acuta and iodide ion for mild steel corrosion in 1 M H2SO4 solutions, Arabian Journal of Chemistry 5 (2012) 325–37. |
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML