-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
International Journal of Materials Engineering
p-ISSN: 2166-5389 e-ISSN: 2166-5400
2014; 4(5): 167-170
doi:10.5923/j.ijme.20140405.01
Transforming Waste Materials as Resources for EAF Steelmaking
Nur Farhana M. Yunos1, Magdalena Zaharia2, Anis Nadhirah Ismail1, Muhammad Asri Idris1
1University Malaysia Perlis, School of Materials Engineering, Arau, Malaysia
2One Steel, Rooty Hill, Sydney, Australia
Correspondence to: Nur Farhana M. Yunos, University Malaysia Perlis, School of Materials Engineering, Arau, Malaysia.
| Email: |  |
Copyright © 2014 Scientific & Academic Publishing. All Rights Reserved.
Agricultural wastes and rubber tires have the potential to be used in industries seeking alternative fuel and sustainable raw materials sources. Previous studies focused on recycling these materials as fuel resources, i.e. rubber in cement industry and agricultural materials for power production. The present paper focuses on investigations of carbon/slag reactions, namely slag foaming and FeO reduction by using rubber and palm shell wastes as sustainable carbon sources through quantitative estimation of the slag volume. An improved volume ratio for the rubber blend and palm shells char compared to coke was seen. It was found that rubber showed a higher rate of FeO reduction than that of coke. However, the present study indicated an opposite trend for palm shell char, where the rate of FeO reduction was found to be lower due to low fixed carbon compared to rubber blend. These results indicate that partial replacement of coke with rubber and palm shell is efficient due to improved interactions with EAF slag.
Keywords: Palm Shells Char, Rubber Tires, Carbon/slag Reactions, FeO Reduction, EAF Steelmaking
Cite this paper: Nur Farhana M. Yunos, Magdalena Zaharia, Anis Nadhirah Ismail, Muhammad Asri Idris, Transforming Waste Materials as Resources for EAF Steelmaking, International Journal of Materials Engineering , Vol. 4 No. 5, 2014, pp. 167-170. doi: 10.5923/j.ijme.20140405.01.
Article Outline
1. Introduction
- Iron and steel making are two of the largest energy intensive industries with the highest growth rate in energy consumption of all energy utilization sectors. In order to meet the growing greenhouse challenges, incorporation of renewable energy sources to the existing and emerging metallurgical operations is desirable. In this respect, agricultural waste from palm shells and rubber tires can potentially be applied in EAF steelmaking as renewable materials. As the chemical components available in these waste materials are carbon and hydrogen, these clearly have the potential to be a cheap and readily available auxiliary source of carbon, to partially replace conventional carbon materials (coke/coal) in steelmaking.The use of plastics as a partial fuel substitute for coke or coal in coke ovens and in the blast furnace is being practiced in Japan [1]. Limited studies have reported the use of polymeric materials in EAF steelmaking and they showed improvements in combustion performances and better carbon/slag interactions with the addition of plastics and rubber [2]. Interactions between FeO containing slag with plastics/coke and rubber/coke blends as a carbon source at 1550 ºC using the sessile drop technique were respectively investigated by Sahajwalla and Magdalena et al. [3-6]. Dankwah et al. [7] measured the reduction of FeO in EAF steelmaking slags using blends of coke, high density polyethylene (HDPE) and rubber tires as reductant and found that the hydrogen and fixed carbon contents leading a faster reduction rates and carburisation.Alternative and renewable materials such as agricultural waste have drawn attention in the iron and steelmaking sector since they are carbon neutral. The use of wood char in ironmaking has been extensively reviewed by Gupta [8], Burgess [9] and Dell’Amico et al [10]. Charcoal has been used as a reducing agent in blast furnaces for a number of years [11], Babich et al. [12] found that the combustion efficiency of all the tested charcoals is better or comparable to conventional coals. However, most of the previous studies considered agricultural materials as reducing agent in the blast furnace, while fewer investigations were considered for applications in steelmaking. Thus, agricultural waste materials derived from palm shells are among the main renewable waste source in Malaysia [13]. The palm shell charcoal was seen to possess other advantageous characteristics; e.g. low sulphur and nitrogen con-tent, resulting in low SOx/NOx emission; the alkaline ash present in the palm shell waste is known to capture some of the SO2 produced during combustion. Besides that, the palm shell waste is a true renewable fuel, so it does not contribute to the increase in the global CO2 concentration.The current paper focus on recycling of wastes derived from polymer and agricultural practices as inject carbon resources in EAF steelmaking industry. It involves a quantitative estimation of the changes occurring in slag volume as a function of time and reduction behavior of FeO slag from the gas formations.
2. Results and Discussion
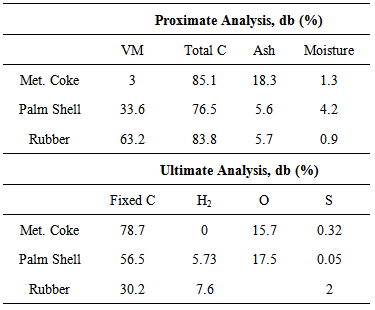
2.1. Materials Characteristics
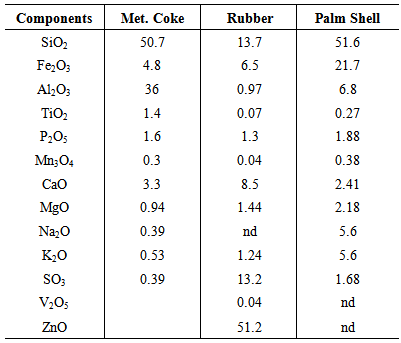
- Metallurgical coke is widely used by steelmakers as the primary carbon resource in EAF steelmaking. The properties of coke, such as fixed carbon content, ash level and composition play an important role in its interactions with molten slag. Rubber is a polymeric material with inherent high volatile content as well as medium amount of carbon (Table 1). Palm shell is a renewable material with high amount of volatiles, low amount of ash which consists mainly of iron oxide and low amounts of sulphur and phosphorus (Table 2). The oxygen in palm shell is available due to its cellulose and hemicellulose content. The presence of oxygen in palm shells may influence CO formation at high temperatures [14].
|
|
|
2.2. Waste Materials (Carbon)/ Slag Interactions
- To qualitatively demonstrate the behavior of the slag volume at steelmaking temperatures, a few representative dynamic wetting images of the slag droplet in contact with metallurgical coke and its blends with rubber and palm shell char are shown in Figure 1. When rubber replaced part of the coke an in-creased slag volume was seen with reaction time. Moreover, with increased reaction time, the volume of the slag in contact with rubber/coke blend was improved as opposed to coke alone. The renewable palm shell showed an increased slag volume compared to coke.
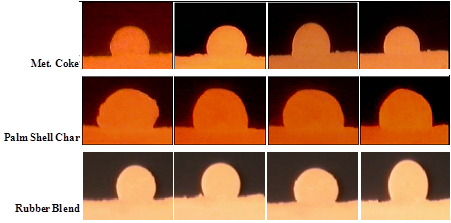 | Figure 1. Snap shots of slag droplets in contact with 100% MC, Rubber Blend, and Palm char at 1550°C as a function of time |
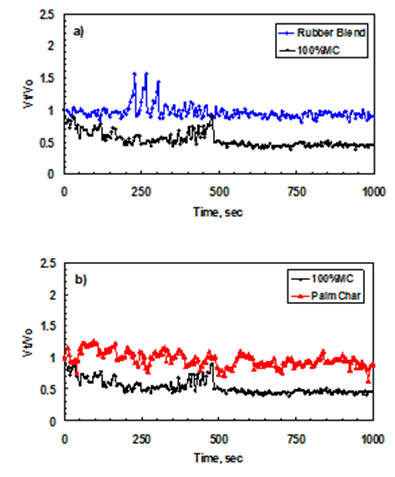 | Figure 2. Vt/V0 plot for coke only and two different rubber/coke ratio blends [15] |
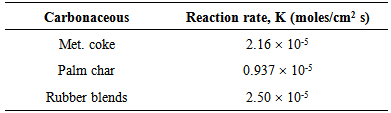
2.3. FeO Reduction
- The rate of reduction is equivalent to the rate of CO and CO2 gases produced from the system. Iron oxide is the main oxide getting reduced as a result of the interaction of slag with various carbonaceous materials under investigations. For metallurgical coke, a value of 2.16 x 10-5 moles/cm2 s was calculated as the maximum rate, while a lower value of 0.937 x 10-5 moles/cm2 s for palm char. On the other hand, rubber blends was measured approximately 2.5 x 10-5 moles/cm2 s (Table 4).
|
2.4. Acknowledgments and Legal Responsibility
- Financial support was provided by FRGS Grant from Ministry of Education, Malaysia.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML