-
Paper Information
- Next Paper
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
International Journal of Materials Engineering
p-ISSN: 2166-5389 e-ISSN: 2166-5400
2014; 4(4): 129-135
doi:10.5923/j.ijme.20140404.02
Microstructure and Mechanical Properties of 3YSZ Ceramics Rainforced with Al2O3 Particles
M. J. Abden1, M. K. Islam2, J. D. Afroze3
1Department of Electrical and Electronic Engineering, International Islamic University Chittagong, Chittagong-4203, Bangladesh
2Department of Electrical and Electronic Engineering, Bangladesh University of Engineering and Technology, Dhaka-1000, Bangladesh
3Department of Applied Chemistry and Chemical Engineering, Noakhali Science and Technology University, Noakhali-3802, Bangladesh
Correspondence to: M. J. Abden, Department of Electrical and Electronic Engineering, International Islamic University Chittagong, Chittagong-4203, Bangladesh.
| Email: |  |
Copyright © 2014 Scientific & Academic Publishing. All Rights Reserved.
The effect of Al2O3 addition on microstructure and mechanical properties of high purity 3 mol% yttria-stabilized zirconia (3YSZ) was investigated. The monoclinic zirconia (m-ZrO2) phase has completely been transformed into tetragonal zirconia (t-ZrO2) phase for Al2O3 content. The presence of 3YSZ particles on the grain boundaries effectively limits a rapid grain growth of the Al2O3 grains. The maximum flexural strength of 340 MPa and Vickers hardness value of 14.31 GPa in the 3YSZ-40 wt% Al2O3 composite were attributed to the microstructure with ZrO2 grains as inter- and intragranular particles in the Al2O3 matrix grains, which makes it suitable for specific dental applications.
Keywords: Zirconia, Alumina, Microstructure, Flexural strength, Vickers hardness
Cite this paper: M. J. Abden, M. K. Islam, J. D. Afroze, Microstructure and Mechanical Properties of 3YSZ Ceramics Rainforced with Al2O3 Particles, International Journal of Materials Engineering , Vol. 4 No. 4, 2014, pp. 129-135. doi: 10.5923/j.ijme.20140404.02.
Article Outline
1. Introduction
- The fabrication of tough and strong ceramics is difficult to achieve due to their inherent brittleness. Zirconia has been one of the most important ceramic materials for well over a century and the discovery of transformation toughening mechanisms in 1975s resulted in improvement of its mechanical properties [1, 2]. Pure zirconia (ZrO2) has three different polymorphs, i. e., monoclinic (m), tetragonal (t), and cubic (c) phases. These phases can be obtained depending on temperature and compositional ranges under equilibrium conditions [3]. The phase transformation of ZrO2 from tetragonal to monoclinic occurs at temperature ~950°C on cooling after sintering, which accompanied by a volume expansion of around 4 vol%, as a result catastrophic failure of the components [2] and, hence, it was very limited interest as a structural and engineering ceramic. However, ZrO2 has other intrinsic physical and chemical properties, including its strength, high toughness, low wear resistance, high elastic modulus, ionic conductivity, chemical inertness as well as high melting temperature that make it attractive as an engineering material and the focus of continued effort to understand and improve its mechanical properties [4-6]. The increase in its industrial applicability has been brought about by the formation of the solid solution to prevent deleterious t → m phase transformation during cooling after sintering. It is well known that a suitable percentage of MgO [7], CeO2 [8] and Y2O3 [9] are most commonly used for this purpose, to retain the tetragonal phase from 1170°C to room temperature, inhibit crack propagation, controlling the extent of stress induced by t → m transformation, and exhibited high fracture toughness [10-12]. Among of them, presently the research efforts appeared to be more focused to the compositions for transformation toughening are those with low yttria concentrations, typically on 3mol% Y2O3 additions yield an extremely fine grained microstructure known as partially stabilized zirconia polycrystals (3YSZ) which exhibited excellent mechanical properties including high toughness, strength and contained only the tetragonal phase at room temperature [13-15]. Thus, this material has been considered as a most potential candidate for load-bearing implant applications, such as dental implants [16, 17]. Cubic zirconia is present in the temperature range of 2370-2680°C. Nevertheless, the c-ZrO2 phase may be obtained at lower temperature upon the addition of above stabilizers [3].However, its poor hardness than other competitive ceramics is ever since main issue. In order to enhance their hardness, it may be dispersed as reinforcement in various composite matrices. Therefore, the combination of a hard ceramic materials such as Al2O3 with a high toughness ZrO2 matrix look as a promising way to produce an excellent composite reinforcement and improve the mechanical properties of the matrix. Besides, the addition of alumina helped to suppress the propagation of phase transformation into the bulk of the material and increased the hydrothermal stability of the tetragonal phase [18, 19]. By the proper combination of particle size and shape grain densification of these composites has been increased [17], which in turn influences on mechanical properties of the ceramics [20]. Likewise, the processing of starting powders, composition, firing time and temperature are also parameters that should be kept in consideration in order to obtain a ceramic free of defects [21, 22]. Two composite materials are prepared based on the ZrO2-Al2O3 system: zirconia matrix reinforced with alumina particles, alumina toughened zirconia (ATZ) or alumina matrix reinforced with zirconia particles, zirconia toughened alumina (ZTA), in both cases, the fracture toughness of the ceramic matrix material as increased [23-25].The purpose of this study is to investigate the influence of Al2O3content on the microstructure and mechanical properties of ZrO2-Al2O3 composites, with an aim to develop ceramic components for dental applications.
2. Experimental Procedure
2.1. Sample Preparation
- In this study α-Al2O3 (99.85% purity, average particle size ~150 nm, Inframat corporation, US) and 3 mol% Y2O3 stabilized ZrO2 (designed 3YSZ, purity 99.9%, average particle size 30-60 nm, Inframat corporation, US) were used as starting powders. Four compositions were prepared by varying the Al2O3 content with 3YSZ from 30, 35, 40, and 45 wt%, respectively. The composite powders were prepared by wet ball-milling for 24 h in ethanol, using high purity zirconia balls (10 mm diameter) as medium to obtain a homogeneous mixture. After milling, the powder mixtures were dried at 90°C for 21 h to remove the ethanol. The powder was crushed gently with the help of mortar and pestle to get proper mixing by adding a few drops of 5 wt% PVA solution as a binder and dried 110°C for 6 h to eliminate the water content of the binder solution. Powder compacts with dimensions of 5 mm × 4 mm × 35 mm were formed by uniaxially pressing at 210 MPaand the green compacts were sintered at 1550 °C for 3 h in air. The heating rate was varied as 8°C/min up to 600°C; 5°C/min up to 1400°C; 3°C/min until the final temperature and sintering for 3 h at the temperature under air atmosphere. The cooling rate was 5°C/min down to until the inertia of the furnace prevailed.
2.2. Characterization Techniques
- The density of the sintered compacts was measured by the Archimedes’ method using distilled water as the immersion medium. The theoretical densities of the composites were estimated by the rule of mixtures, using a theoretical density of 3.99 gm/cm3 for Al2O3 and 6.09 gm/cm3 for 3YSZ, respectively. The crystalline phases of the composite specimens was conducted by a D8 ADVANCE X-ray diffractometer (Bruker AXS, Karlsruhe, Germany) with Cu-Kα radiation (λ = 0.15406 nm) in a continuous mode from 2θ range 20-90° at an angular step of 0.02° and a fixed counting time of 0.6 s/step. The voltage and current were set at 40 kV and 40 mA, respectively. The average grain size of the ZrO2 and Al2O3 grains was obtained by analyzing scanning electron microscopy (SEM) images of randomly selected areas of the fracture surfaces using the linear intercept method. Flexural strength was determined using three point bending test on five bars for every specimen by universal tensile tester (Hounsfield H10K-S, UK) with the inner span set to 25 mm and a velocity of the cross head displacement of 0.5 mm/min. The hardness of the sintered samples was evaluated at room temperature by a micro-Vickers hardness tester (Shimadzu, HMV-2, Japan) with an indent load on polished surfaces of 19.614 N held for 6 s. The measurement was repeated 12 times for a sample and the average value was determined in which the maximum and minimum values were excluded.
3. Results and Discussion
3.1. X-ray Diffractometry of Powders
- The phases of the as-received 3 mol% Y2O3 stabilized ZrO2 (3YSZ) powder was investigated by means of XRD and the respective pattern is illustrated in Figure 1. It was observed that the main phase is tetragonal (t) as well as few amount of monoclinic (m) phase of ZrO2 was observed, which characterized by diffraction peak approximately at 2θ = 28° and 31° and some of cubic (c) phase with reflections in 2θ = 74.54° [26].
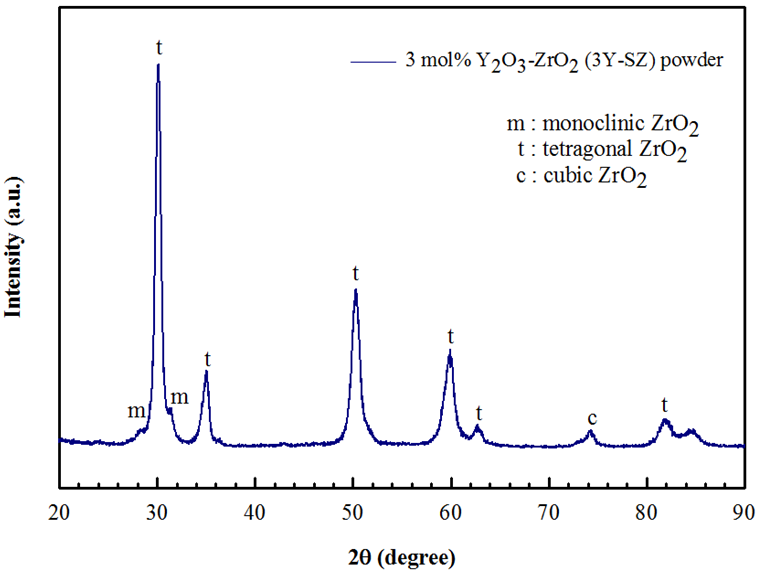 | Figure 1. X-ray diffraction pattern of as-received 3YSZ powder |
3.2. Sintering Behavior and Microstructure
- Figure 2 shows the variations of relative density for homogeneous mixtures with different percentages of Al2O3 (30, 35, 40, and 45 wt%) with 3YSZ after sintering during 3 h at 1550°C in air. It can be observed that the maximum sintered relative density (>98%) was attained for the composition 3YSZ with additions of 30 wt% Al2O3 content. It is important to focus that the relative density decreases with increasing the Al2O3 content, indicating that the addition of Al2O3 suppressed the densification of 3YSZ matrix. These results are in good agreement with the findings of Zhang et al. [6] who reported that the relative density decreases as the Al2O3 content increases. Figure 3 presents micrographs of the ZrO2-Al2O3 composites with varying Al2O3 contents sintered at 1550°C in 3 h. The micrograph exhibits the Al2O3 and ZrO2 grains as dark and whitish color respectively, where zirconia grains are embedded around the alumina grain and/or within the grain. However, small fractions of intragranular zirconia grain are also being noticed. Figure 3(a) shows that the zirconia grains eventually coalesce with each other are located at grain junctions and are responsible for a grain boundary pinning effect. Higher volume fraction of zirconia causes the zirconia to be less isolated and more continuous and interconnected. Figure 3(b) and (d) micrographs shows relatively more isolated pores and these pores were interconnected in some occasions. Micropores are present at the triple junctions of the ZrO2 grains. The presence of pores tends to increase the diffusion distance between particles thereby reducing the driving force for pore shrinkage. In this context, the final relative density of the compositions was lower than the other compositions. Figure 3(c) microstructure reveals that the zirconia particles are isolated at grain boundaries between bigger alumina grains and some isolated pores were observed. Most of the ZrO2 grains were located at the triple junctions of the Al2O3 grains and the grain boundaries must be due to the strong self-diffusion of zirconia, which revealed the intergranular nature of the ZrO2 grains. Although some small ZrO2 grains were located within the Al2O3 grains and had spherical geometry, possessing the intragranular type.
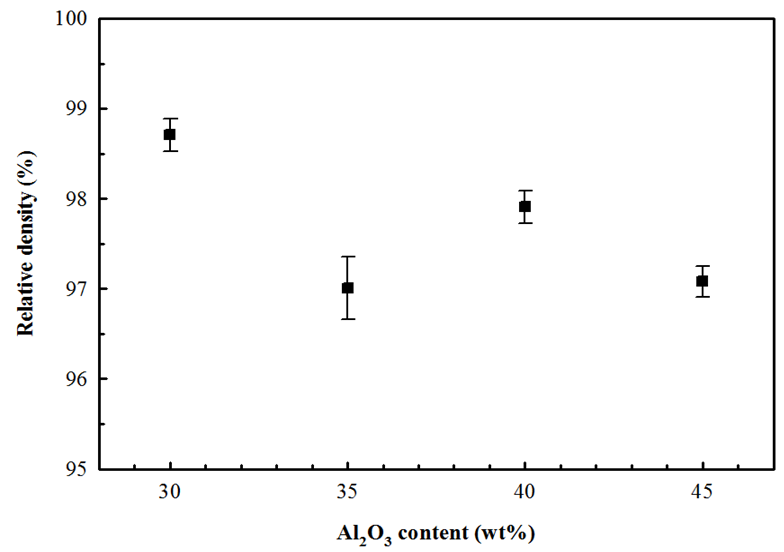 | Figure 2. Relative density of 3YSZ-Al2O3 composites as a function of Al2O3 content |
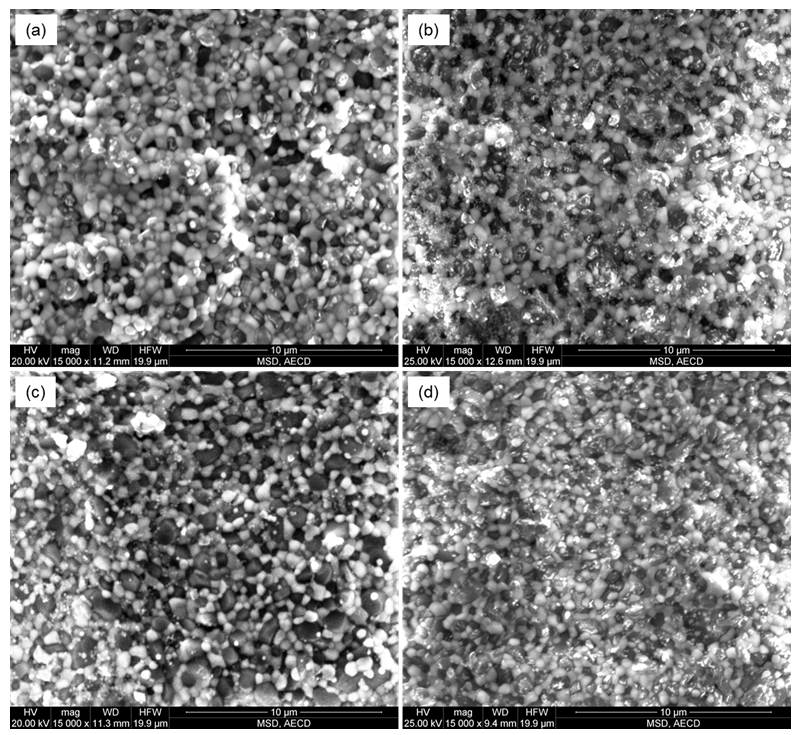 | Figure 3. SEM micrographs of fracture surfaces of (a) 3YSZ-30 wt% Al2O3, (b) 3YSZ-35 wt% Al2O3, (c) 3YSZ-40 wt% Al2O3, and (d) 3YSZ-45 wt% Al2O3 composites sintered at 1550°C for 3 h in air |
3.3. Phase Analysis
- Figure 4 shows the X-ray diffraction patterns of different samples sintered at 1550°C for 3 h. In these patterns, it was observed that the main phase is tetragonal (t) as well as a reflection in 2θ = 74.54° to the (200) peak belonging to the c-ZrO2 phase and a few amount of monoclinic (m) phase was observed only for the composition of 30 wt% Al2O3 content, which is characterized by diffraction peak approximately at 2θ = 28° and 31°. But for higher Al2O3 content there is no trace of monoclinic (m) phase of ZrO2, exhibit a dual microstructure, formed by t-ZrO2 and c-ZrO2 grains, which confirmed that the m-ZrO2 phase content in the starting powder has completely been transformed into tetragonal phase, indicating the complete stabilization of the tetragonal phase forhigher Al2O3 content [27]. It is also seen that the peak intensities of α-Al2O3 reflections in the XRD patterns increases continuously, indicating to the increase in weight fraction of Al2O3 in the ZrO2 matrix.
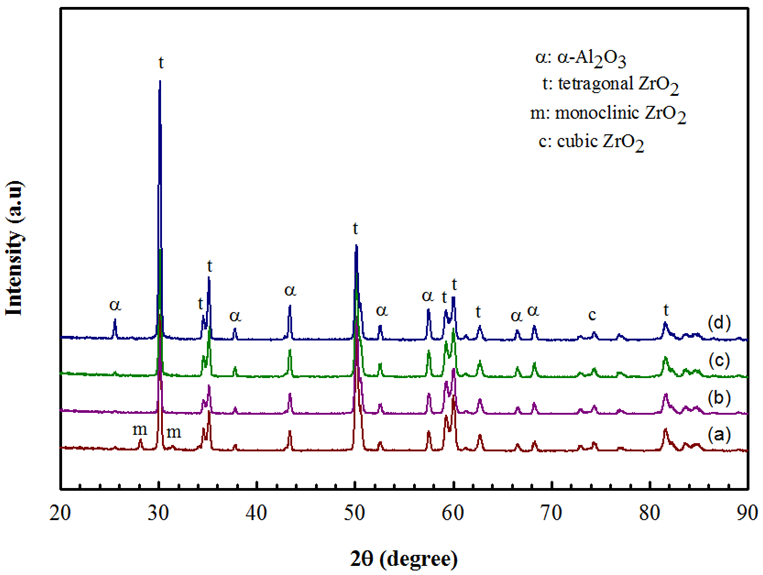 | Figure 4. XRD patterns of (a) 3YSZ-30 wt% Al2O3, (b) 3YSZ-35 wt% Al2O3, (c) 3YSZ-40 wt% Al2O3,(d) 3YSZ-45 wt% Al2O3 bulk samples sintered at 1550°C for 3 h in air |
3.4. Mechanical Properties
- Figure 5 shows the results of the three-point bending tests as a function of the concentration of alumina particles in the zirconia matrix. Despite the errors associated to these measurement, it is observed that the higher flexural strength valueof 340 MPa in ATZ system were archived for the composition containing 40 wt% Al2O3, which are very similar compared to the strength values reported in the literature using spark plasma sintering [28].
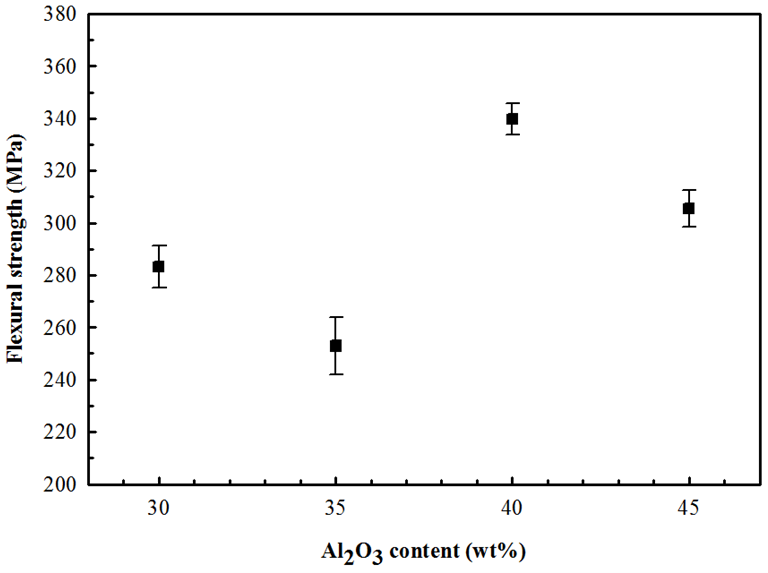 | Figure 5. Flexural strength of 3YSZ-Al2O3 composites as a function of Al2O3 content |
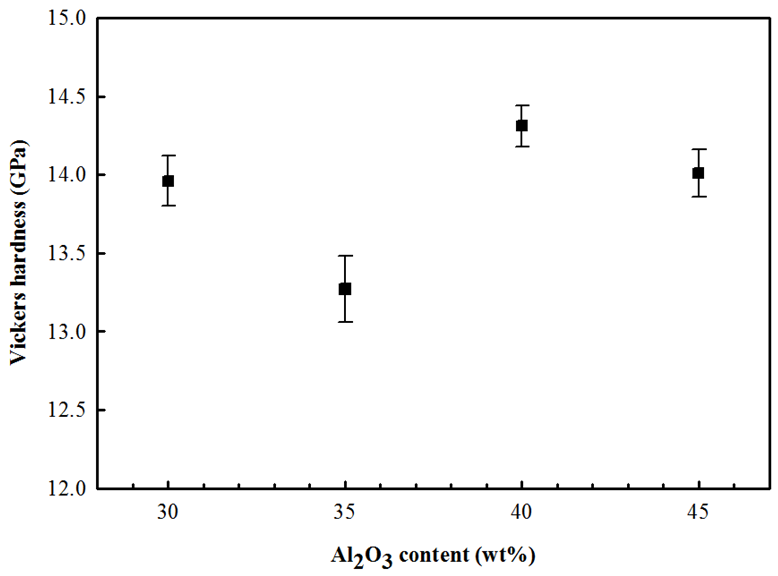 | Figure 6. Vickers hardness of 3YSZ-Al2O3 composites as a function of Al2O3 content |
4. Conclusions
- In all sintered materials, it was found that the main phase is tetragonal (t) as well as a few amount of monoclinic (m) phase was observed only for 3YSZ-Al2O3 composite material, containing 30 wt% of Al2O3, while for higher Al2O3 content exhibited a dual microstructure formed by tetragonal and cubic phases, indicating the m-ZrO2 phase content has completely been transformed into t-ZrO2 by Al2O3 content. The sintering characteristics and mechanical properties are strongly dependent on the distribution of second phase of Al2O3. The presence of 3YSZ can pin the grain boundaries and inhibit the grain growth of Al2O3, making the refinement of the microstructure possible. The 3YSZ-40 wt% Al2O3 composites of highly dense microstructure with ZrO2 grains as inter- and intragranular particles in the Al2O3 matrix grains, shows improved flexural strength (340 MPa) and Vickers hardness (14.31 GPa), which in turn should have the ideal combination of mechanical properties for dental applications.
ACKNOWLEDGEMENTS
- The authors grateful to the authority of Bangladesh Council of Scientific and Research, Dhaka-1205 and Materials Science Division, Bangladesh Atomic Energy Center, Dhaka-1207 for permitting to use their laboratories.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML