Traoré S.1, Diakité S.2, Traoré D. L.2
1Department of Chemical Engineering, University of Conakry, UGANC, Guinea
2Department of Civil Engineering, University of Conakry, UGANC, Guinea
Correspondence to: Traoré S., Department of Chemical Engineering, University of Conakry, UGANC, Guinea.
| Email: |  |
Copyright © 2012 Scientific & Academic Publishing. All Rights Reserved.
Abstract
Guinea is home to 33 percent of the world’s known bauxite reserves and is the fourth largest producer and second largest exporter of bauxite. The extraction and processing of metallic ore deposits for minerals, metals production are strongly related to the generation of enormous quantities of solid wastes, which cause serious environmental damages on the air, soils and water resources. In Guinea, the ACG plant produces 600000 metric tons of alumina per year and generates an equal quantity of red mud (RM). This paper deals with the geoplymerization of the red mud and metakaolin (MK) in order to develop geopolymeric materials(GMK) with advanced mechanical properties. Some structural aspects of the resulting products were studied according to X-ray Diffraction analysis, and Scanning Electronic Microscopy. The effect of the synthesis parameterson the compression strength and leaching test values of the GMK products were investigated. The inorganic polymeric materials produced by the geopolymerization of the red mud developeda good compressive strength. Samples with leachate concentrations close to 100 ppm exhibit the best mechanical performances.
Keywords:
Bauxite, Red mud, Metakaolin, Geopolymer
Cite this paper: Traoré S., Diakité S., Traoré D. L., Investigation of a Red mud and Metakaolin-based Inorganic Polymer Material for Civil Construction Applications, International Journal of Materials Engineering , Vol. 4 No. 1, 2014, pp. 25-30. doi: 10.5923/j.ijme.20140401.03.
1. Background
1.1. Bauxite Resources in Guinea
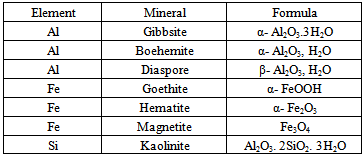
Bauxite is a member of the family of lateritic rocks. It is characterized by a particular enrichment of aluminum- hydroxide minerals. Alumina occurs in 3 phases defining ore type: gibbsitic (γ-Al(OH)3), boehmitic (γ-AlO(OH)) and diasporic (α-AlO(OH)). The mineral gibbsite, termed "hydrargillite" in European literature, is commonly referred to as an alumina trihydrate, Al2O3·3H2O, because chemical analyses of the mineral indicate a ratio of one molecule of alumina (Al2O3) to three molecules of water. The atomic structure of gibbsite, however, contains no water molecules, and all water detected by analyses is in hydroxyl (OH) form. Gibbsite is, therefore, more correctly an aluminum trihydroxide, Al(OH)3[1].Guinea is home to 33 percent of the world’s known bauxite reserves and is the fourth largest producer and second largest exporter of bauxite.An assessment of the bauxite resources of Guinea claims a quantity of 40 billion metric ton comprising 3178 billion metric tons of proven and probable deposits, 7381 gauged resources, 18686 metric tons indicated and supposed resources, an additional 10895 metric ton forecasted reserves [2].Table 1. Mineral forms of some elements present in bauxite
 |
| |
|
The most important bauxite mining operations in Guinea are Compagnie des Bauxites de Guinée (CBG) with an annual capacity of 14,000,000 tonnes, Compagnie des Bauxites de Kindia (CBK)with an annual capacity of 3,000,000 tonnes; and Alumina Company of Guinea with an annual capacity 2,800,000 tonnes[3].The Fria bauxite deposit is located in western part of the Guinea Republic, between N10"20'-10"30' and W13"30'-13"40. Within the general area of bauxitization there are about 20 small ore deposits, each containing between 2 and 20 Mt. The deposit lies at the junction of the Konkouré and Badi rivers, both of which deeply incise surrounding plateaus (200-260 m) and hills (280-320 m). These lateritic bauxites, located on the upper parts of plateaus, result from weathering of paleozoicschists. The ores are composed of gibbsite associated with pyrophyllite, Al-substituted goethite, and kaolinite[4].These are high in alumina (56-60% Al2O3), and have Fe2O3 contents ranging from 1-8Y0 and SiO2 contents ranging from 1-12%.The Fria bauxite deposits formed on the Paleozoic cover of the West African Craton, which was uplifted during the Tertiary along the Guinean axis. Deposits are mostly developed on Gothlandianschists which are enclosed by two thick Ordovician and Devonian sandstone series[5].
1.1.1. Alumina Production in ACG-Fria
Basically the alumina production in ACG plant is the Bayer process that remains the most economical for alumina extraction. During this process, the insoluble product generated after bauxite digestion with sodium hydroxide at elevated temperature and pressure to produce alumina is known as red mud “RM “or “bauxite residue”. The waste product derives its color and name from its iron oxide content.
1.1.1.1. Chemical Reactions in Bayer Process
A major problem is the dissolution in caustic soda liquor of silica; the silica arises from kaolinit a constituent of bauxite | (1) |
 | (2) |
The basic digestion reaction is the following: | (3) |
Depending on the specific raw mineral:  | (4) |
 | (5) |
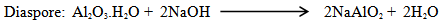 | (6) |
Crystalline alumina hydrate is extracted from the digestion liquor by hydrolysis. | (7) |
1.1.1.2. Bauxite and Alumina Wastes in Guinea
It is estimated that approximately 35% - 40% per ton of bauxite treated ends up as waste via the Bayer process. Furthermore, about 70 million tons of bauxite residue or RM are produced yearly worldwide and are not utilized[6]. In Guinea, the terminology used by the 3 main bauxite mining industries CBG, ACG and CBK is “bauxite mud” and in that of alumina extraction, “red mud”.Bauxite mud may be found in quarries as well as in the installations of the plants treating bauxite. Investigations suggest that wherever there is dust in the dry season, there will be mud during the winter season. The mud comes most frequently from washing the machines (conveyor belts, feeding chains of the crushing mill) and from the meeting points of the feeding conveyors for the dryers. The most important problem is experienced at the plant at Kamsar because of the large quantity of bauxite that is treated there each day[7].The Friguia plant rejects one ton of mud per ton of alumina produced; each ton of mud contains in average 15 kilograms of soda (NaOH) no recovered by washing; this mud is composed on average of 60% iron ore (Fe2O3), 30 % lime (CaCO3) with traces of titanium (TiO2). On the basis of 600000 metric tons of alumina production per year, ACG Fria generates an equal quantity of RM. Over the company’s 30 years of existence, the quantity of RM produced may very well exceed 20 million metric tons. It should be mentioned that until the end of the 1980’s this mud was simply dumped in the Konkouré river which flows not far from the plant.
1.2. Geoplymers
The geopolymer technology has recently attracted increasing attention as a viable solution to reuse and recycle industrial solid wastes and by-products. It provides a sustainable and cost-effective development for many problems where hazardous residues have to be treated and stored under critical environmental conditions. Geopolymers appear to be apotential alternative to the classic hydraulic binders. Furthermore, geopolymers have the advantage to be possibly formulated from a wide range of aluminosilicate minerals, as from industrial wastes. Generally, materials containing mostly amorphous silica (SiO2) and alumina (Al2O3) are a possible source for geopolymer production. This diversity in material sources places it as interesting solution for RM incorporation.The geopolymerization mechanism involves Siand Al dissolution from the starting materials generates to make available polysialate units (e.g., sialate[_Si_O_Al_O], sialatesiloxo[_Si_O_Al_O_Si_O] or sialatedisiloxo [_Si_O_Al_O_Si_O], depending on the Si/Al ratio) cross-linked[AlO4]_ and [SiO4] tetrahedral units, with charge balance ensured by Na+ or K+ ions[8]. The sialate is an abbreviation for silicon-oxo-aluminate. There is also an empirical formula for geopolymer matrix:M+n{-(SiO2)z-AlO2-}nWhere M+ = an alkalicathion (K+, Na+) for balancing the negative charge of Al3+ in IV-fold coordination; n = degree of polymerization; and z = Si/Al ratio. By varying the Si/Al ratios (i.e., z = 1-15, up to 300)[9]. The developed formula of oligomer units can be represented as follows: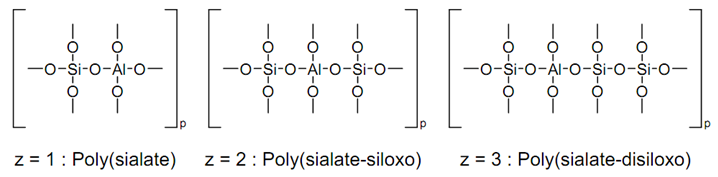
2. Materials and Method
- The red mud (RM) whose chemical composition was taken from RMpiles of the ACG alumina plant in Fria; the chemical analysis was carried out in ACG Laboratory.- Technical grade kaolin was purchased on free market in Conakry; it was subject to dehdroxylation in the temperature range between 650 and 850°C in order to produce metakaolin as source of aluminosilicate. The resulting metakaolin is predominantly an amorphous material with minor crystalline constituents.- Sodium hydroxide pro analysis(Sigma-Aldrich Co., USA) - Sodium silicate solution pro analysis(Merck; Germany) with Na2O =8%, SiO2 = 27%, H2O = 65% and d = 1,346 g/I.- Deionized water made in Pharmaguinee Laboratories. The sodium hydroxide was dissolved in deionized water and then mixed with the sodium silicate solution to obtain the aqueous phase. The blend was mixed by on a Heidolph ST-1 Laboratory stirrer. Then the RM was mixed with MK in the following MK/RM compositions: 1/4; 1/6; 1/8; 1/10; resulting with GMK4, GMK6, GMK8 and GMK10 as final products.The two phases were mixed on a mechanical mixer for 5 minutes to produce homogeneous pastes that was transferred into moulds and cured under atmospheric pressure at 60°C for 3 days. Then samples were demoulded and the presumed geopolymers products (GMK4, GMK6, GMK8 and GMK10) were left certain time at ambient temperature for more hardening before properties investigations.The X-ray diffraction was conducted on a Rigaku Geigerflex D/max – Series instrument. The microstructural characterization was carried out by scanning electron microscopy (SEM – Hitachi, SU 70) and energy dispersive X-ray spectrometry (EDS – EDAX with detector BrukerAXS, software: Quantax). Metallic cubic moulds (50x50x50 mm) for the specimens of compressive strength test and plastic cylindrical moulds (30 x 50 mm) for the specimens of leaching tests.The compressive strength was measured on a Shimazdu apparatus (Model: AG-X/R Refresh).
3. Results and discussion
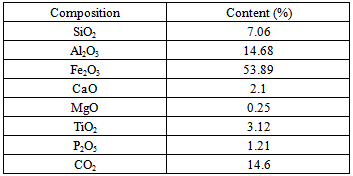
Table 2. Main chemical constituents of RM from the ACG alumina plant
 |
| |
|
Tab.2 shows the composition of RM from ACG alumina plant. It has a high rate of iron compound in the shape of hematite and goethite. The aluminum hydroxide (mainly gibbsite etboehmite) from non-recovered or unreacted Al2O3 represents about 15%. Mineralogically, iron content is found as hematite (Fe2O3) or goethite (FeOOH), while aluminum content is found as gibbsite (Al2O3·3H2O), diaspore (Al2O3·H2O), cancrinite (NaAlSiO4)6CaCO3 and katoite (Ca3Al2(SiO4)(OH)8. Sodium originated from caustic soda as lixiviatingagent.The red mud contains also titanium mainly inthe form of rutile, which is a common accessory mineral in bauxite deposits along with some amorphous compound.Numerous toxic metals accumulate in these muds in important concentrations in the following order[10]: Cr, Mn, Pb, Sr, Ba, Mo, Sb, Bi, Zn, Co, Ag, As, Li, and Cd. Thus, RMitself does not provide the geopolymeric system with the appropriate soluble Si and AI. Soluble silicate in the geopolymeric systems is an essential factor for the geopolymerization process, affecting directly its efficiency. MK is rich in easily dissolved silicon and aluminum.The X-ray diffraction analysis shows that the dominating minerals in both RM and MK are quartz (Q) and kaolinite (K). The X-ray diagrams indicate that the treatment is characterized by dissolution of the starting material and a formation of amorphous and crystalline aluminosilicate phases as well as the stable phases of leucite and kalsilite.Geopolymers are often described as ‘X-ray amorphous [11], since the major feature of powder X-ray diffraction patterns is a ‘featureless hump’ centered at approximately 27–29°2Ɵ.The XRD patterns of investigated GMK geopolymers show a broad reflection related to the high amorphous content, like that observed for metakaolin. Nevertheless, the center of this reflection is shifted to 2Ɵ=29° due to changes in composition and structure when metakaolin is activated by NaOH and NaSiO2 solutions. This amorphous alkaline aluminosilicateis the dominant product, corresponding to 90 %in all samples. The Scanning Electron Microscopy of the investigated samples Fig.2(a), shows that the microstructure of geopolymer produced by mixture of RM and MK comprises non-dissolved particles of RM, which are bonded in an extent gel phase and Fig5(b) the formation of gel silicate phase (Si > 40% and Fe < 7%).The reaction with the alkaline solution to form a particulate gel network took place at the border of particles then involving the entire surface.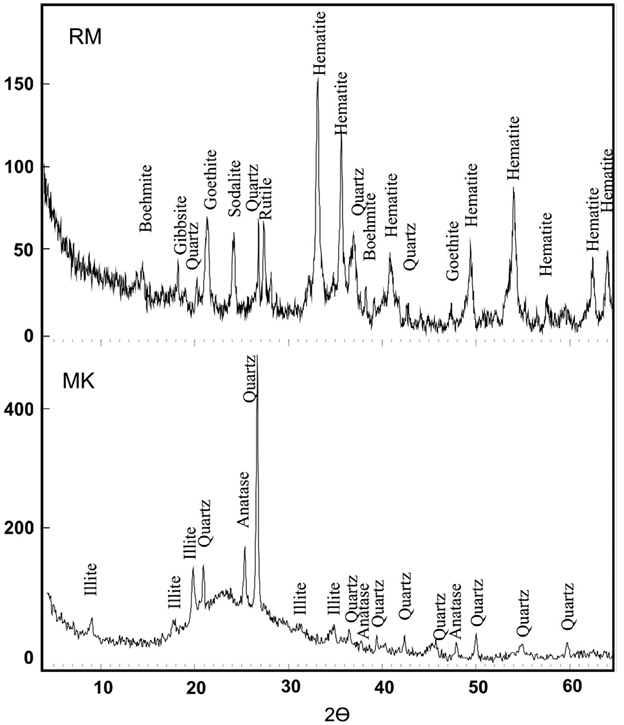 | Figure 1. X-ray diffractograms of red mud and metakaolin samples |
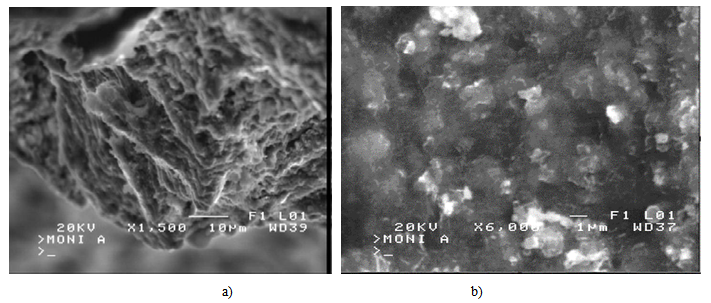 | Figure 2. SEM micrographs of GMK geopolymer show non-dissolved particles of red mud and gel phase |
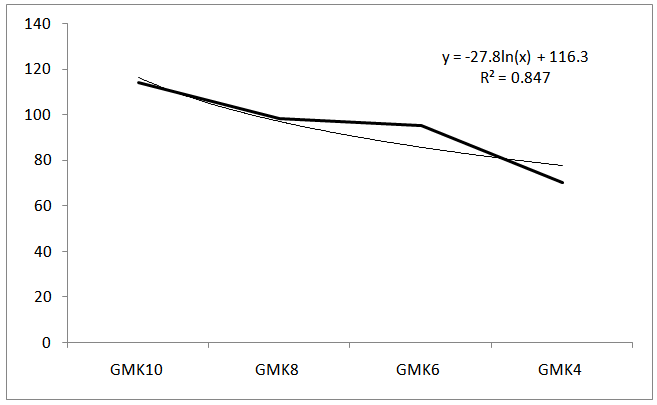 | Figure 3. Sodium leaching values (ppm) |
Several studies claim that the solid to liquid (S/L) ratio and the sodium hydroxide and silicon concentrations in the aqueous phase are the main synthesis parameters affecting the physical and mechanical properties of geopolymers[12]. The red GMK materials developed compressive strength between 3.8 and 9.5 MPa, as S/L ratio was increased from 2 to 3.1gml-1. The compressive strength showed initial maximum values after 24 hours curing around 8MPa. The amount of RM seems to have variable effect on the mechanical properties of geopolymer. The resistance of sample GMK4 decreased to minimum values below 5MPa. Hence, the lower the SiO2/Al2O3 ratio, the weaker is the strength of geopolymer.By growing the RM content, also the Na2O/SiO2 ratio increased and a higher strength was measured for the 1/10, 1/8 and 1/6 ratios, which showed that the compressive resistance is maximal when the of Na2O/Al2O3 molar ratios are between 1 and 3. This confirmed by the work of Stevenson[13]. Over and below this window, as it occurs in GMK4, the polymerized network is less stable and easily disintegrated.Fig.3. shows the leaching test of GMK products. Correlation exists between leaching results and compressive strength values. Samples with leachate concentrations close to 100 ppm exhibit the best mechanical performances. However, both low (GMK4 and GMK8) leachate concentrations result in low strength, this trend might be related also to variation of sodium content in initial formulations. Due to the high porosity, sodium forming part of the geopolymer network is easily extractable by Na+ and H+ exchange in aqueous media.
4. Conclusions
The above considerations on the bauxite resources of Guinea show that the resulting red mud as waste from the Bayer process represents an environmental threat and a technological challenge. Red mud utilization is an opportunity to develop promising engineering applications; such as its incorporation in construction materials.Inorganic polymer or geopolymer was designed by sodium silicate/NaOH activation of mixtures of metakaolin and red mud. It shows the feasibility of chemically bonded materials. Sodium hydroxide and silicate solution contents were proved as crucial synthesis parameters of geopolymers for the development of mechanical properties, as they affect directly or indirectlyall the stages of the geo-polymerization process.The inorganic polymeric materials produced by the geopolymerization of the red mud and metakaolin developed a good compressive strength. Samples with leachate concentrations close to 100 ppm exhibit the best mechanical performances.
References
| [1] | Newsome, J. W., Heiser, H. W., Russell, A. S., and Stumpf, H. C., (1960): Alumina properties: Alcoa Research Lab. Tech. Paper 10, 88 p. |
| [2] | Mamedov, Dr V.(2003): Catalogue des gisements et indices de minéralisation bauxitique en République de Guinée. |
| [3] | United States Geological Survey (various issues), “Bauxite Statistics and Information”, Washington, D.C.: USGS |
| [4] | B. Boulangé, G. Bouzat, M. Pouliquen (1996): Mineral. Deposita 31, 432-438 ; Springer-Verlag |
| [5] | B. Boulangé et al(1996):Mineral. Deposita 31, 432-438. |
| [6] | Mamadou Saliou Diallo, “Les instruments juridiques et le développement durable du secteur de la bauxite en Guinée”. Master’sthesis in EnvironmentalStudies, Université du Québec à Montréal, October 1995. |
| [7] | Giannopoulos I, Pania D. (2007):Structure, design and applications of geopolymeric materials. In: third international conference on deformation processing and structure of materials, BelGMKade, Serbia. |
| [8] | MacKenzie et al. (2006): Mackenzie, K. J. D., Brew, D., Fletcher, R., Nicholson, C., Vagana, R, and Schmucker, M. (2006): “Advances in understanding the synthesis mechanisms of new geopolymeric materials.” Novel Processing of Ceramics and Composites, 195, 187-200. |
| [9] | WaghArun S.(1986): A Study of Jamaican Bauxite Waste, Report 154 (vol. I – III) to International Development and Research Centre, Ottawa, Canada, University of the West Indies, Kingston, Jamaica, , pp.171. |
| [10] | Patel, C.B. Jain, V.K. and Pandley G. S.: (1986) “Micro Polluants in RM Waste of Aluminium Plants”, inInternational Journal of Analytical Chemistry, vol. 25, pp 269-274. |
| [11] | Granizo ML, Alonso S, Blanco-Varela MT, Palomo A (2002): J Am Ceram Soc 85:225. |
| [12] | Palomoet al.(1999): Swanepoel and Strydom, 2002; Van Deventer et al., 2002; Cheng and Chiu, 2003; Panias et. al., 2007. |
| [13] | Steveson M, Sagoe-Crentsil K.: (2005): Relationships between composition, structure and strength of inorganic polymers; Part 2: Flyash-derived inorganicpolymers. J Mater Sci.; 40:4247–59. |





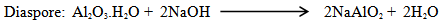





 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML
