| [1] | M. E. Zorn, D. T. Tompkins, W. A., Zeltner, and M. A. Anderson, 2000, Catalytic and Photocatalytic Oxidation of Ethylene on Titania-Based Thin-Films., Environ. Sci. Tech., 34(24), 5206-5210. |
| [2] | M.E. Manriquez, T. Lopez, R. Gomez, and J. Navarrete, 2004, Preparation of TiO2-ZrO2 mixed oxides with controlled acid-basic properties., J. Mol. Catal. A-Chem., 220, 229-237. |
| [3] | X. Fu, L.A. Clark, Q. Yang, and M.A. Anderson, 1996, Enhanced Photocatalytic Performance of Titania-Based Binary Metal Oxides: TiO2/SiO2 and TiO2/ZrO2., Environ. Sci. Tech., 30(2), 647–653. |
| [4] | L. Valdez – Castro J. Méndez – Vivar, and R. Mendoza – Serna, 2001, Porous SiO2–TiO2–ZrO2 Obtained from Polymeric Systems Prepared by the Sol–Gel Process, J. Porous Mat., 8, 303 – 309. |
| [5] | T. Ivanova, A. Harizanova, T. Koutzarova, and B. Vertruyen, 2011, Preparation and characterization of ZnO–TiO2 films obtained by sol-gel method., J. Non-Cryst. Solids, 357, 2840–2845. |
| [6] | G. Marci, V. Augugliano, and M.J. López-Muňoz, 2001, Preparation, characterization and photocatalytic activity of polycrystalline ZnO/TiO2 systems., Surface and bulk characterization, J. Phys. Chem. B, 105, 1026-1040. |
| [7] | S. Liao, Y. Donggen, D. Yu, Y. Su, and G. Yuan, 2004, Preparation and characterisation of ZnO/TiO2, SO42-/ZnO/TiO2 photocatalyst and their photocatalysis., J. Photochem. Photobiol. A, 168(1-2), 7–13. |
| [8] | A. Sclafani, L. Palmisano, and M. Schiavelo, 1990, Influence of the preparation methods of titanium dioxide on the photocatalytic degradation of phenol in aqueous dispersion., J. Phys. Chem., 94(2), 829–832. |
| [9] | T. Lopez, R. Gomez, E. Sanchez, F. Tzompantzi, and L. Vera, 2001, Photocatalytic Activity in the 2,4-Dinitroaniline Decomposition Over TiO2 Sol-Gel Derived Catalysts., J. Sol-Gel Sci. & Techn., 22( 1-2), 99-107. |
| [10] | M. Gärtner, V. Dremov, P. Müller, and H. Kisch, 2005, Bandgap widening of titania through semiconductor support interactions., ChemPhysChem, 6(4), 714-722. |
| [11] | N.F. Uvarov and V.V. Boldyrev, 2001, Size effects in the chemistry of heterogeneous systems., Usp. Khim., 70(4), 307-329. |
| [12] | A.A. Gribb and J. F. Banfield, 1997, Particle size effects on transformation kinetics and phase stability in nanocrystalline TiO2., Amer. Mineral, 82 (7-8), pp. 717-728. |
| [13] | S.J. Smith, R. Stevens, S. Liu, G. Li, A. Navrotsky, J. Boerio-Goates, and B. F. Woodfield, 2009, Heat capacities and thermodynamic functions of TiO2 anatase and rutile: Analysis of phase stability, Amer. Mineral., 94(2-3), 236-243. |
| [14] | P. Yang, D. Zhao, D.I. Margolese et al., 1998, Generalized syntheses of large-pore mesoporous metal oxides with semicrystalline frameworks, Nature (London), 396, 152–155. |
| [15] | R. Vogel, P. Meredith, I. Kartini, M. Harvey, J. Riches, A. Bishop, N. Heckenberg, M. Trau, and H. Rubensztein-Dunlop, 2003, Mesostructured Dye-Doped Titanium Dioxide for Micro-Optoelectronic Applications., ChemPhysChem, 4, 595-603. |
| [16] | Yu. Gnatyuk, N. Smirnova, A. Eremenko, and V. Ilyin, 2005, Design and Photocatalytic activity of mesoporous TiO2/ZrO2 thin films., Ads. Sci. & Techn., 23, 497-503. |
| [17] | І. Petrik, O. Кеlyp, V. Vorobets, N. Smirnova, О.Frolova, О. Оranska, G. Kolbasov, and A. Eremenko, 2011, Synthesis, optical, photo- and electrocatalytic properties of nanosized TiO2 modified with transition metal ions., Chem., Phys. & Techn. Surf., 2(4), 436–442. |
| [18] | Yu. I. Gnatyuk, V. I. Yatskiv, N. P. Smirnova, V. M. Granchak, and A. M. Eremenko, 2005, Photocatalytic properties of mesoporous TiO2/ZrO2 films in gas-phase oxidation of alcohols., Theoret. Experim. Chem., 41(6), 371-376. |
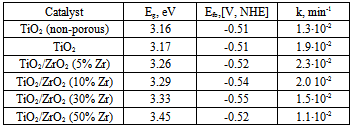
| [19] | Yu. Gnatyuk, N. Smirnova, O. Korduban, and A. Eremenko, 2010, Effect of zirconium incorporation on the stabilization of TiO2 mesoporous structure., Surf. Interface Anal., 42, 1276 – 1280. |
| [20] | E. Manujlov, Yu. Gnatyuk, N. Smirnova, A.Eremenko, V. Vorobets, G. Kolbasov, A. Guobiené, and S. Tamulevicius, Mesoporous TiО2 and TiО2/ZnО/Ag films: sol-gel synthesis, photoelectrochemical and photocatalytic properties., P. Innocenzi, Yu. Zub, V. Kessler Eds. Springer, 2008. |
| [21] | T. Ptashko, N. Smirnova, A. Eremenko, E. Oranska, and W. Huang, 2007, Synthesis and photocatalytic properties of mesoporous TiO2/ZnO films with improved hydrophylisity., Ads. Sci. & Techn, 25, 35-43. |
| [22] | N. Smirnova, V. Vorobets, O. Linnik, E. Manuilov, and G. Kolbasov, 2010, Рhotoelectrochemical and photocatalytic properties of mesoporous TiO2 films modified with silver and gold nanoparticles., Surf. Interface Anal., 42, 1205 – 1208. |
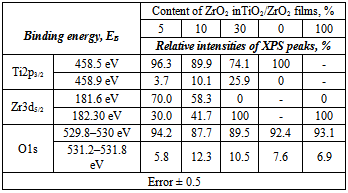
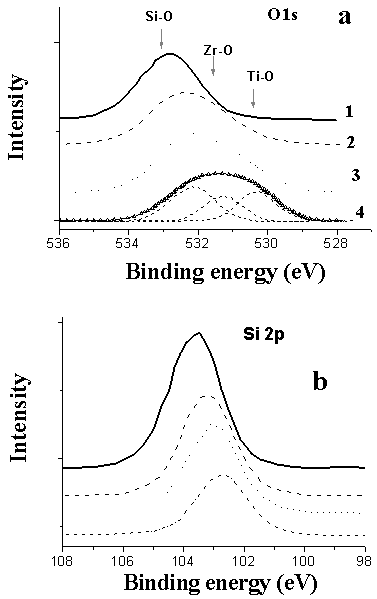
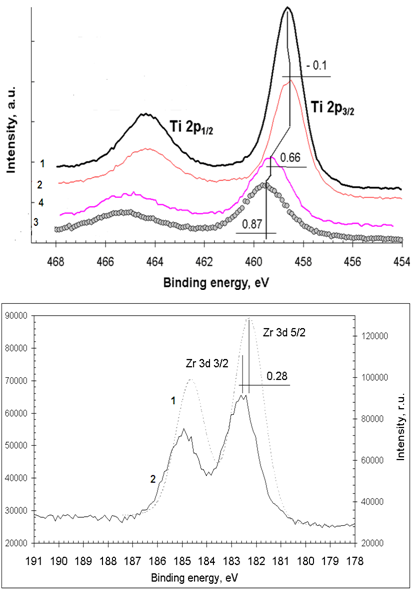
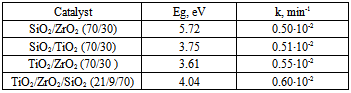
| [23] | M. Andrulevičius, S. Tamulevičius, Yu. Gnatyuk, N. Vityuk, N. Smirnova, and A. Eremenko, 2008, XPS investigation of TiO2/ZrO2/SiO2 films modified with Ag/Au nanoparticles., J. Mat. Sci. (MEDŽIAGOTYRA), 149(1), 8 – 14. |
| [24] | N.V. Vityuk, A.М. Eremenko, N.P.Smirnova, P.P. Gorbik, Т. Busko, М.P. Kulish, О.P. Dmitrenko, V.V. Shlapatska, and V.А. Khizhny, 2007, Effect of irradiation on structural and photocatalytic properties of nanosized TiO2/ZrO2/SiO2 composites., Phys. Chem. Solid State, 8(4), 776 – 781. |
| [25] | O. Linnik and H. Kisch, 2006, On the mechanism of nitrogen photofixation at nanostructured iron titanate films., Photochem. Photobio. Sci., 5, 938-942. |
| [26] | N. Smirnova, Yu. Gnatyuk, A. Eremenko, G. Kolbasov, V. Vorobetz, I. Kolbasova, and O. linyucheva, 2006, Photoelectrochemical characterization and photocatalytic properties of mesoporous TiO2/ZrO2 films., Intern. J. Photoenergy, 8, 1-6. |
| [27] | S.I. Gregg and K.S.W. Sing, Adsorption, Surface Area and Porosity” Academic Press, London, 1982. |
| [28] | C.C. Hyun, M.J. Young, and B.K. Seung, Size effects in the Raman spectra of TiO2 nanoparticles., Vibrational Spectr., 37, 33–38, 2005. |
| [29] | C.D.Wagner, J. F.Moulder, L. E. Davis, W. M. Riggs, Handbook of X-ray Photoelectron Spectroscopy, Perking - Elmer Corp.: New York, AQ4 1979. |
| [30] | Tanabe K., Catalysis: Science and Technology. Anderson JR, Boudart M Eds. Springer, New York, 1981. |
| [31] | V. Vorobets, E. Manujlov, N. Smirnova et al., 2008, Electro- and photocatalytic properties of electrodes based on mesoporous TiO2-ZnO-Ag films., Chem., Phys. & Techn. of Surf., 14, 382-390. |
| [32] | U. Siemon, D. Bahnemann, J. J. Testa, D. Rodríguez, M. I. Litter, and N. Bruno, 2002, Heterogeneous photocatalytic reactions comparing TiO2 and Pt/TiO2., J. Photochem. Photobio. A, 148(1–3), 247–255. |
| [33] | G. Colon, M. C. Hidalgo, and J. A. Navio, 2001, Photocatalytic Deactivation of Commercial TiO2 Samples During Simultaneous Photoreduction of Cr(VI) and Photooxidation of Salicylic Acid., J. Photochem. Photobio., A , 138(1) 79-85. |
| [34] | J. Kim, K.C. Song, S. Foncillas, and S.E. Pratsinis, 2001, Dopants for synthesis of stable bimodal porous titania., J .Eur. Ceram. Soc., 21, 2863-2872. |
| [35] | A. Naumenko, Iu. Gnatiuk, N. Smirnova, and A. Eremenko, 2012, Characterization of sol–gel derived TiO2/ZrO2 films and powders by Raman spectroscopy., Thin Solid Films, 520(14), pp. 4541–4546. |
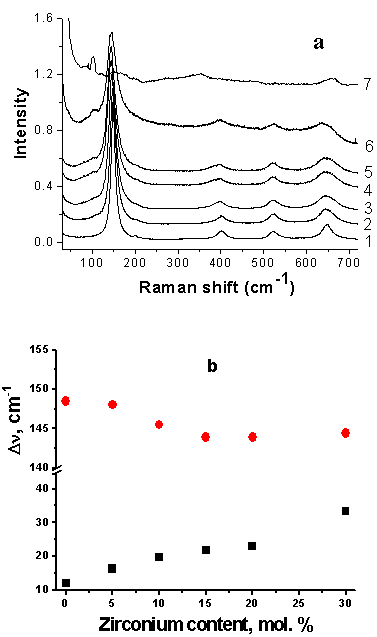
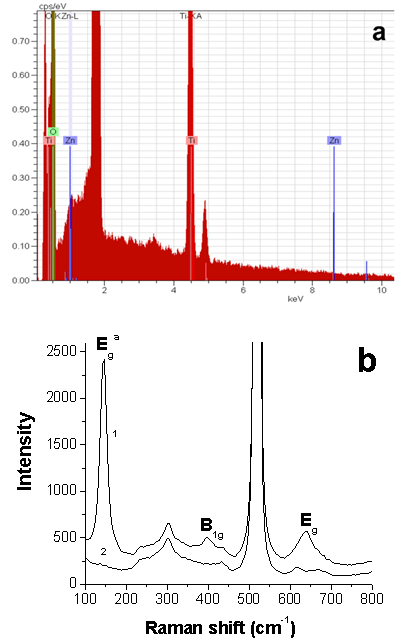
| [36] | T. Ohsada, F. Izumi, and Y. Fujiki, 1978, Raman spectrum of anatase., TiO2, J. Raman Spectrosc., 7(6), 321-324. |
| [37] | J.C. Yu, J. Lin, and R.M.W. Kwok, 1998, Ti1-xZrxO2 solid solutions for the photocatalytic degradation of acetone in air., J. Phys. Chem. B, 102, 5094 – 5098. |
| [38] | M. Durr, S. Roselli, A. Yasuda, and G. Nelles, 2006, Band-gap engineering of metal oxides for dye-sensitized solar cells, J. Phys. Chem. B, 110(43), 21899- 21902. |
| [39] | Nefedov V.I., Roentgenoelectronic spectroscopy of chemical compounds, Chemistry, Moskow, 1984. |
| [40] | Yu. Ja. Gurevitch and Yu. V. Pleskov, Photoelectrochemistry of Semiconductors, Nauka, Moscow, 1983. |
| [41] | A. L. Linsebigler, G. Lu, and J. T. Yates, 1995, Photocatalysis on TiOn Surfaces: Principles, Mechanisms, and Selected Results., Chem. Rev., 95, 735-758,. |
| [42] | S. Sakthivel and H. Kish, 2003, Photocatalytic and photoelectrochemical properties of nitrogen-doped titanium dioxide., ChemPhysChem, 4. 487-490. |
| [43] | M. Gratzel, S. E. Gilbert, C. Klemenz, and H. J.Scheel, 1996, Electrochemical and photoelectrochemical investigations of single-crystal anatase., J. Amer. Chem. Soc., 118, 716-6723. |
| [44] | Ye. V. Kuzminskii and G. Ya. Kolbasov, 1999, Electrochemical systems for converting solar energy., Solar Ener. Mat. Solar C., 5, 93-115. |
| [45] | Chen, Shi-Fu, and Xue-Li Cheng, 1999, Photocatalytic Reduction of Dichromate by Titanium Dioxide Supported on Hollow Glass Microbeads., Chin. J. Chem., 17(4), 419-24. |
| [46] | U. Diebold, 2003, The surface science of titanium dioxide., Surf. Sci. Reports, 48, 53 – 229. |
| [47] | J.-Chr. Buhl and A. Willgallis, 1989, Hydrothermal synthesis of (Zr0.33 Ti0.67) O2-srilankite in the system ZrO2-TiO2-H2O-MF; (M = Na, K)., Cryst. Res. Techn., 24, 263 – 268. |
| [48] | U. Troitzsch, A.G. Christy, and D.J. Ellis, 2005, The crystal structure of disordered (Zr,Ti)O2 solid solution including srilankite: evolution towards tetragonal ZrO2 with increasing Zr., J. Phys. Chem. Minerals, 32, 504 – 514. |
| [49] | W.F.Zhang, Y.L.He, M.S.Zhang, Z.Yin and Q.Chen, 2000, Raman scattering study on anatase TiO2 nanocrystals., J. Phys. D: Appl. Phys, 33, 912 – 916. |
| [50] | F.M.Liu, B.Ren, J.H.Wu, J.W.Yan, X.F.Xue, B.W.Mao, and Z.Q.Tian, 2003, Enhanced Raman scattering from silicon nanoparticle substrates., Chem. Phys. Lett., 382, 502-507. |
| [51] | S.N.Tkachev, M.H.Manghnani, A.Niilisk, J.Aarik, and H.Mändar, 2005, Raman and Brillouin scattering spectroscopy studies of atomic layer-deposited ZrO2 and HfO2 thin films., Spectrochim. Acta A, 61, 2434-2438. |
| [52] | C. D. Wagner, A. V. Naumkin, A. Kraut-Vass, J. W. Allison, C. J. Powell, and J.R. Rumble Jr. NIST Standard Reference Database 20, Version 3.4 |
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML