-
Paper Information
- Next Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
International Journal of Materials Engineering
p-ISSN: 2166-5389 e-ISSN: 2166-5400
2013; 3(2): 11-16
doi:10.5923/j.ijme.20130302.01
Performance of TiO2/In(OH)iSj/Pb(OH)xSy Composite ETA Solar Cell Fabricated from Nitrogen Doped TiO2 Thin Film Window Layer
C. O. Ayieko1, R. J. Musembi1, S. M. Waita1, B. O. Aduda1, P. K. Jain2
1Department of Physics, University of Nairobi, P.O. Box 00100-30197, Nairobi, Kenya
2Department of Physics, University of Botswana, P/ Bag 0022, Gaborone, Botswana
Correspondence to: C. O. Ayieko, Department of Physics, University of Nairobi, P.O. Box 00100-30197, Nairobi, Kenya.
| Email: |  |
Copyright © 2012 Scientific & Academic Publishing. All Rights Reserved.
In this work, Titanium Dioxide (TiO2) thin films were prepared by spray pyrolysis and thermally annealed at 400℃. The films were characterized as deposited (no annealing) as well as after annealing. Optical studies showed that the energy band gap of the films was lowered from 3.25 eV to 2.90 eV on Nitrogen (N2) doping. The reduction in energy band gap was attributed to the introduction of N2 impurity states on the bands (conduction band and or valence band). The effect of N2 doping of Titanium Dioxide window layer on the efficiency of the ETA TiO2/In(OH)iSj/Pb(OH)xSy solar cell was investigated using a conventional current-voltage (I-V) technique. The photovoltaic conversion efficiency (η) increased from 1.06% for the solar cell with undoped films to 1.32% for the solar cell with N2-doped films. The increase in photovoltaic conversion efficiency on doping was attributed to increased light absorption due to the Nitrogen doping.
Keywords: Titanium Dioxide, Conversion efficiency, Doping
Cite this paper: C. O. Ayieko, R. J. Musembi, S. M. Waita, B. O. Aduda, P. K. Jain, Performance of TiO2/In(OH)iSj/Pb(OH)xSy Composite ETA Solar Cell Fabricated from Nitrogen Doped TiO2 Thin Film Window Layer, International Journal of Materials Engineering, Vol. 3 No. 2, 2013, pp. 11-16. doi: 10.5923/j.ijme.20130302.01.
Article Outline
1. Introduction
- Meeting human energy requirements has been an elusive task even after the discovery of the immense energy from the atom when the phrase “too cheap to meter” was coined[1] and the energy cost has been soaring each passing day. The energy crisis in the 1970’s which made the cost of petroleum almost quadruple necessitated intensive research and development on renewable energy sources[1].Solar energy, one of the renewable sources, is environmentally friendly. It is also universal and versatile with the ability to be made available even to the remotest parts of the world. Solar energy systems do not involve heavy moving parts and hence present minimal wear and tear and therefore require very low maintenance once installed. The initial cost of installation for solar energy systems is high just as for other energy sources but due to low maintenance costs, it guarantees free energy after a short payback period. This makes solar energy systems viable[2].Solar cells convert solar energy into electrical energy. Different technologies have been developed to fabricate solar cells, and the Extremely Thin Absorber (ETA) technique is one of the promising methods. The concept of ETA solar cells involves using an extremely thin absorber layer of less than 50 nm. Some promising characteristics of ETA solar cells include: reduced charge carrier transport time through the solar cell due to thin absorber, enhanced photon absorption due to scattering resulting from the porous nature of the window layer[3]. A major challenge facing ETA solar cells is low photoelectric conversion efficiencies. TiO2 has been used as window layer for both all-solid state and dye sensitized solar cells. A porous TiO2 deposited by spray pyrolysis was used as a window layer in the fabrication of TiO2/In(OH)iSj/Pb(OH)xSy/PEDOT:PSS ETA solar cell and it was observed that the highly porous TiO2 ensured increased absorption of incident radiation through scattering[4]. Studies on N2-doped TiO2 deposited by the pressing technique for dye sensitized solar cells (DSSC) application have been done[5]. However, the challenge encountered with the DSSC solar cell was leakages from the liquid electrolyte. Gartner et. al[6] have also done some studies on N2-doped TiO2 thin films and reported an increase in light harvesting towards the visible range. However, they never used the doped films in any specific application. Although some work has been done for N2-doped TiO2, to the best of our knowledge, N2 doping of TiO2 has not been done for spray pyrolysis deposited TiO2 films and applied to the ETA solar cell. This work therefore investigates the performance of an all-solid state ETA solar cell with TiO2 thin films deposited by spray pyrolysis followed by N2 doping, that is, N:TiO2/In(OH)iSj/Pb(OH)xSy solar cell.
2. Experimental
- Fluorine-doped tin oxide coated glass substrates (FTO) were cleaned in an ultrasonic bath filled with distilled water for 2 minutes before deposition. The substrates were then rinsed in acetone and finally rinsed in distilled water. Deposition of TiO2 by spray pyrolysis was done by mixing 120 µl of commercially available Titanium (iv) Isopropoxide iC12H28O4) (purity 99.7%) and 200 ml Isopropanol (C3H8O) (purity 99.7%) from Fluka Ltd. The mixture was heated to 50℃ using an electric hot plate and the temperature maintained constant while stirring with a magnetic stirrer for about 15 minutes. The mixture formed the precursor which was used for spray pyrolysis. Nitrogen gas was used as the carrier gas. The spray nozzle-to-substrate distance was kept at about 15.0 cm. The substrate temperature (Ts) was varied from 100℃ to 200℃ for different samples. Coating was done by spraying in pulses. A pulse consisted of 5 seconds of spraying and 30 seconds of pause. Ten pulses were done for every sample at a precursor flow rate of 2.6 ml/min. The chemical reaction resulting in the formation of amorphous TiO2 thin film was as follows:
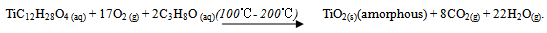 | (1) |
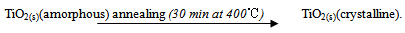 | (2) |
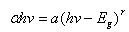 | (3) |
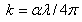 | (4) |
2.1. Deposition of Indium Hydroxy Sulphide (In(OH)iSj) buffer Layer
- Nitrogen-doped and undoped TiO2 thin films were coated with Indium Hydroxy Sulphide buffer layer by chemical bath deposition (CBD) in a solution prepared using commercially obtained reagents from Sigma Aldrich Ltd and prepared as follows: 20000 ± 1µl of distilled water was mixed with 1250 ± 1µl of 0.005M of Hydrochloric acid (HCL) which was then mixed with 1250 ± 1µl of 0.005M Indium (III) Chloride (In2Cl3) and 2500 ± 1µl of 0.1M Thioacetamide (CH3CSNH2). The resulting solution was kept in a water bath that was maintained at a temperature of 70 ± 1℃. The TiO2 films were dipped into the solution prepared above in turns, each dip lasting 30 minutes. The films were then rinsed in distilled water to clean off unwanted chemical remnants. The procedure was repeated three times for each film. Finally the films were annealed at a temperature of 300℃ in air.
2.2. Deposition of Lead Hydroxy Sulphide (Pb(OH)xSy) Coating
- Lead Hydroxy Sulphide (Pb(OH)xSy) coating was deposited by successive ion layer adsorption and reaction (SILAR). In this process, saturated Lead Acetate (Pb(CH3OOH)2) solution (97% pure) and Sodium Sulfide (Na2S) solution (97% pure) both commercially obtained from Sigma Aldrich Ltd. were used. To deposit Lead Hydroxy Sulphide (Pb(OH)xSy) coating, the following four-stage process was followed: First the films were submerged in lead acetate solution for 10 seconds for the metal ions to be adsorbed then rinsed in distilled water Secondly, they were submerged in Sodium Sulfide solution for 10 seconds to allow Sulphur ions to react with metal ions and finally were rinsed in distilled water. The procedure constituted one cycle giving a particular thickness.
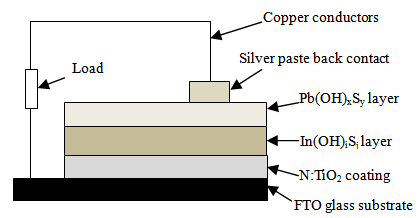 | Figure 1. Schematic cross-section of the N:TiO2/In(OH)iSj/Pb(OH)xSy composite (ETA) solar cell |
2.3. I-V Characterization
- Current-voltage (I-V) characterization was done using a solar simulator equipped with a 0.5kW Xenon arc lamp and a 1.5 AM Global Air Mass Filter. Illumination was maintained at 1 sun (1000 W/m2). The solar cell area was 0.1256 cm2.
3. Results and Discussion
3.1. Structural Analysis
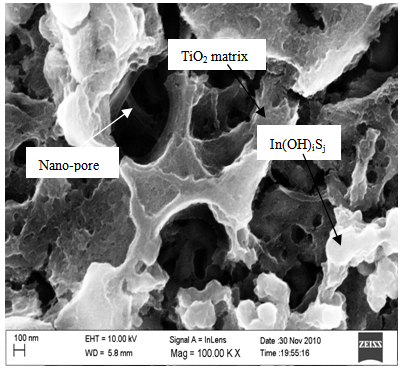
- Figure 2 shows a SEM micrograph of Nitrogen-doped TiO2 and Indium Hydroxy Sulphide (Pb(OH)xSy) layer. The TiO2 is porous with nano pore sizes of approximately 500 nm. This morphology is favourable for light scattering effect and increases the light optical path, thus enhancing light absorption which makes the films appropriate for solar cell applications.
3.2. Optical Characterization
3.2.1. Reflectance, Transmittance and Absorption
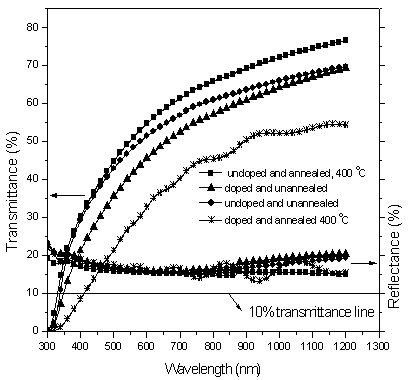
- Figure 3 shows transmittance and reflectance characteristics for undoped and Nitrogen-doped TiO2 films (both annealed and unannealed). Except the doped and annealed film (which is thicker compared to all other films), the other three films are of comparable thickness as seen from the transmittance and reflectance spectra. Generally, thicker films tend to have lower transmittance than thin films. The lower transmittance observed can therefore be attributed partly to the film’s thickness. To find out whether doping plays any role, we compare the undoped and unannealed and the doped and unannealed films since they are of similar thickness. It is observed that the doped and unannealed has a lower transmittance (22-55%) compared to (34-60%) for the undoped and unannealed in the visible range-400-800 nm and this can be attributed to the presence of the dopant in the film since all the other conditions are the same. It can therefore be concluded that doping also contributes to the low film transmittance (10-45% in the visible range-400-800 nm) in the doped and annealed film.
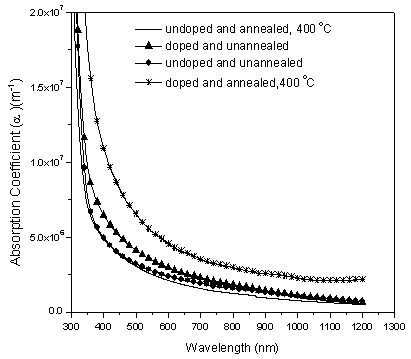 | Figure 4. Dependence of absorption co-efficient on wavelength of Nitrogen doped and undoped TiO2 thin films deposited on fluorine doped tin oxide (FTO) glass slides |
3.2.2. Effect of Nitrogen-doping on the band gap of TiO2 Coated on Fluorine Doped Tin Oxide (FTO) Glass Slides
- Figure 5 shows the estimated band gap of the films coated on Fluorine doped Tin Oxide (FTO) substrate and annealed at 400℃. The band gap decreases from 3.25 eV for the undoped film to 2.90 eV for Nitrogen-doped. Our results are in agreement with those obtained by Baoshun[9] who also observed a decrease in the band gap TiO2 prepared by sputtering from 3.2 eV to 2.7 eV upon Nitrogen doping. This reduction is as a result of nitrogen impurity states which introduce tail energy levels either in the conduction band or valence.
3.3. TiO2/In(OH)iSj/Pb(OH)xSy ETA Solar Cell Performance
3.3.1. J-V characterization
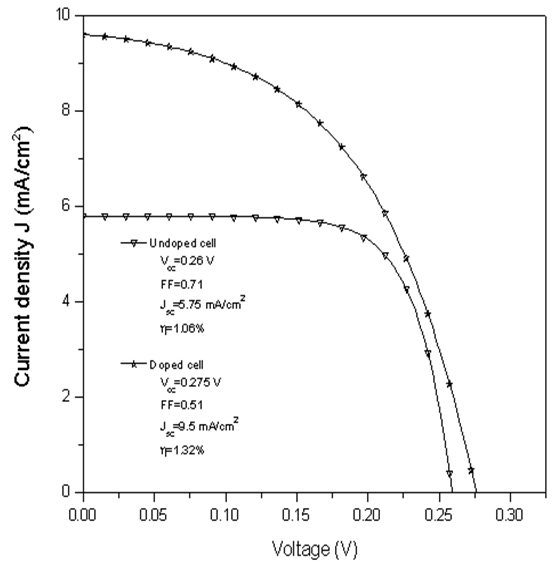
- The current density versus voltage graph for the fabricated solar cells is shown in Figure 6.The ETA solar cell fabricated from the doped TiO2 film shows superior performance. The current density increases by a factor of 1.65 while the efficiency increases by a factor of 1.24. The improved performance of the ETA solar cell on doping can be attributed to the increased light absorption due to Nitrogen doping.
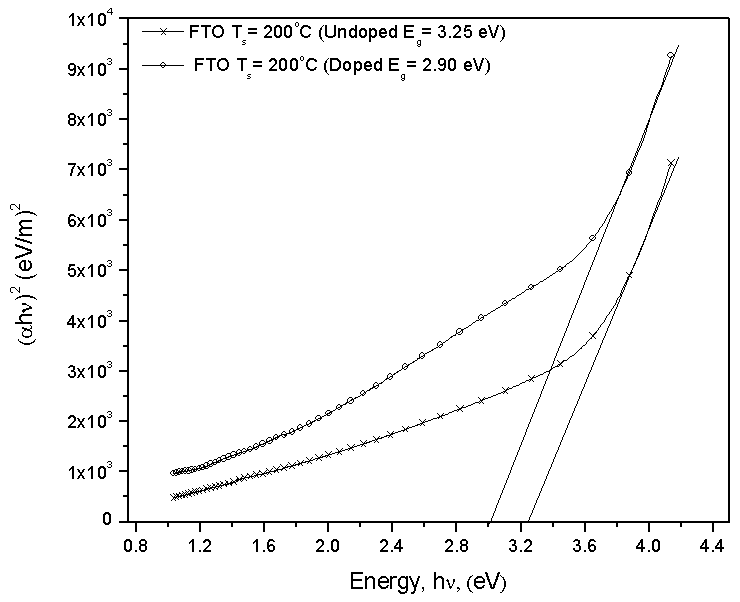 | Figure 5. Graph showing the effect of doping TiO2 thin film coated on conducting flourine doped tin oxide (FTO) glass slides with Nitrogen (N2) and annealed at 400℃ |
4. Conclusions
- Doped and undoped TiO2 thin films have been prepared by spray pyrolysis. X-ray diffraction studies shows that the as prepared films are transformed from amorphous to crystalline after annealing at 400℃. The films were confirmed to be porous through SEM images. Optical studies showed that Nitrogen doping increases the light absorption of the films by lowering the energy band gap from 3.25 eV to 2.90 eV. ETA solar cells fabricated from doped TiO2 films had better photovoltaic performance than those from undoped films. The current density increases by a factor of 1.65 while the efficiency increases by a factor of 1.24. The improved performance of the ETA solar cell on doping can be attributed to the increased light absorption due to energy band gap lowering through Nitrogen doping. It has been established that spray pyrolysis and the doping technique (heat treatment) used in this paper are viable options for the preparation of Nitrogen doped TiO2 films.
ACKNOWLEDGEMENTS
- The authors thank African Materials Science and Engineering Network (AMSEN) and International Programme in the Physical Sciences (IPPS), Uppsala University (Sweden) for financial support.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML