S K Shukla1, M. Deepa2, Santosh Kumar1
1Steel Product Group, Research and Development Centre for Iron and Steel, Steel Authority of India Limited, Ranchi, 834002, India
2Physical Metallurgy Group, Research and Development Centre for Iron and Steel, Steel Authority of India Limited, Ranchi, 834002, India
Correspondence to: S K Shukla, Steel Product Group, Research and Development Centre for Iron and Steel, Steel Authority of India Limited, Ranchi, 834002, India.
| Email: |  |
Copyright © 2012 Scientific & Academic Publishing. All Rights Reserved.
Abstract
In order to improve the corrosion resistance of the conventional hot dip galvanized sheets, a new type of Zn-Mg coating has been developed. Experiments were conducted in HDPS by varying zinc bath Mg level, bath temperature & dipping time. It has been found that steel sheet galvanized in zinc bath composition of 0.50%Mg, 0.25%Al, 0.08%Si & 0.08%Sb and processed at bath temperature of ~460oC and dipping time : 2.0 sec. results in best combination of properties in terms of corrosion rate : 4.0 mpy (~1/3rd w.r.t conventional GI sheets : ~12 mpy), formability of the composite (Ev : 10.4 mm for sheet thickness of 0.8 mm) at par with substrate material, coating adherence as per Lock Forming Quality (LFQ) standard and appearance bright and smooth. The improved corrosion resistance of newly developed coating is attributed to the presence of Mg-Zn intermetallic phases along the grain boundary inhibiting the cathodic reaction as well as formation of protective MgO & protective corrosion products of zinc. The presence of Sb in the galvanizing bath has also led to improved intergranular corrosion resistance. Superior formability, adherence and appearance of the coating is due to presence of adequate Al & Si level in the galvanizing bath, which led to suppression of brittle Fe-Zn phase formation at the coating-steel interface.
Keywords:
Zn-Mg Coating, Corrosion Resistance, Formability, Coating Simulation
Cite this paper: S K Shukla, M. Deepa, Santosh Kumar, Effect of Mg Addition (in Zinc Bath) on Galvanized Sheet Quality, International Journal of Materials Engineering, Vol. 2 No. 6, 2012, pp. 105-111. doi: 10.5923/j.ijme.20120206.05.
1. Introduction
Zinc coated sheets are widely used in Construction, Automotive and appliance industry. The demand by the customers for the performance of the coated sheets with respect to corrosion protection, formability, joinability and paintability is steadily increasing. The demand for extended longevity of the galvanized sheet is increasing to meet the demand for maintenance free products. To meet this demand the corrosion resistance of the galvanized sheets need to be improved. Corrosion resistance of the zinc coated products can be improved by alloying the galvanizing bath with aluminium, nickel, magnesium and other alloying elements. Addition of aluminium to zinc bath is one of the most useful development so far. Nevertheless, there is scope for further development in terms of coating parameter, composition and lower bath temperature to improve corrosion resistance and other properties, and at a lower cost so that it can be economically exploited. As regards to alloying elements, in addition to Al, several reports have published the effect of Mg[1,2]. Mg has the best corrosion resistance, lowest density among metals, superior specific strength and high electro-negativity in emf series. Also, Zn-Mg alloys can offer low bath temperature[3,4]. Further, It has also been reported that small amount of Si addition in zinc bath improves the coating ductility [5]. In view of the above, the aim of the present work was to find out the optimum level of Mg in zinc bath as well as optimised galvanizing parameters such as bath temperature, dipping time etc. for achieving improved properties in terms of corrosion resistance, formability properties along with adequate peel-off resistance of the zinc coating. In addition to above, for improving the intergranular corrosion resistance of the Zn-Mg-Al-Si coating, possibility of replacing Pb with Sb in zinc bath has also been explored. This will also help in addressing the environmental issues related with Pb.
2. Experimental
During coating simulation experiments, Commercial Quality Cold Rolled (CQCR) sheets (thickness : 0.8 mm) were galvanized in HDPS by varying zinc bath Mg level (0.25-0.75%), bath temperature (420-460oC) & dipping time (2.0-4.0 sec.), while Al, Si & Sb levels were maintained at ~0.25%, ~0.08% & ~0.08% respectively. Chemical composition of the steel substrate used in simulation studies has been shown in Table I. Process variables and their ranges have been shown in Table II. | Table 1. Chemical composition of cold rolled substrate used in Simulation Studies |
| | Element | Wt (%) | | C | 0.06 | | Mn | 0.30 | | Si | 0.030 | | S | 0.012 | | P | 0.015 | | Al | 0.030 | | N | 50 ppm |
|
|
| Table 2. Process variables and their ranges during simulation experiments |
| | S.No. | Process variable | Range | | 1. | Mg level in zinc bath | 0.25-0.75% | | 2. | Bath Temp. | 420-460oC | | 3. | Dipping Time | 2.0-4.0 sec. |
|
|
In simulation tests, CQCR sheets of size 200 mm (L) x 120 mm (W) were heated at the rate of 30oC/s to annealing temperature of 750oC and soaked at this temperature for 45 sec. in annealing atmosphere of 20%H2+80%N2. Dew point of annealing atmosphere was kept at ~ -20oC during experimentation. Subsequent to annealing, samples were cooled with N2 gas upto near bath temperature (5oC more than the bath temperature) at the rate of ~4oC/s and subsequent to dipping in the zinc bath, samples were cooled at the rate of 10oC/s till room temperature.Experimental galvanized sheet samples were characterized in terms of coating thickness, corrosion rate, formability, coating adherence & microstructures. Coating Thickness (CT) of the galvanized samples was measured using Defalsko Coating Thickness Gauge (Model : Positector 6000). Formability characteristics of GI sheets were evaluated through Erichsen Cup Tester. During erichsen cup test, the point at which the cracks/peel-off of the coating begins, punch movement at that point was taken as the erichsen cup value of the composite. Coating adherence of the coated sheets was assesses through Lock Forming Tester. Corrosion characteristics of GI sheets were assessed through Taffel plot using potentiostat under the following conditions : Test Solution : 3.5% NaClReference Electrode : Silver – Silver chloride.Scan Rate : 0.1 mv/s.Scan Range : ± 20 mv.Metallographic analysis of simulated GI sheet samples was carried out using Scanning Electron Microscope attached with EDAX system.
3. Results & Discussion
Simulated galvanized sheets were characterized in terms of coating thickness, Corrosion rate, formability properties and coating adherence. The results have been shown in Table III.
3.1. Corrosion Resistance of Zn-Mg Coated Sheets
Corrosion resistance of the Zn-Mg-Al-Si coated sheets, measured through electropolarisation test using Taffel plot, varied in the range of 4.0 to 10.5 mpy (compared to 12-15 mpy observed in conventional zinc coated sheets), depending on the processing condition. For the coated sheets, galvanized in 0.25% Mg bath, corrosion rate varied from 8.0 to 10.5 mpy., while the corrosion rates of the galvanized sheets of 0.50 & 0.75% Mg bath was found in the range of 4.0 to 7.6 mpy. In Fig.1, variation of corrosion rate with Mg content is shown. Table 3. Properties of Zn-Mg coated sheets
 |
| |
|
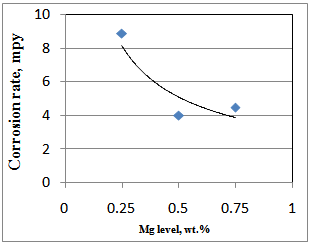 | Figure 1. Effect of Mg on corrosion rates of Zn-Mg coated sheets |
It can be seen that with increase in Mg content from 0.25 to 0.75% for a bath temperature of 460oC and dipping time of 2.0 sec., corrosion rate of the Zn-Mg coated sheets decreases from 10.5 to 4.0 mpy. From the above it can be observed that increase in Mg content from 0.25 to 0.50% improves the corrosion rate by more than 50%, however, further increase of Mg level from 0.50% to 0.75% doesn’t results in any further improvement in corrosion rate of the coated sheets. Corrosion rates of the coated sheets galvanized in 0.75%Mg bath was found more or less same as that of the 0.50%Mg bath. High corrosion resistance of the material coated in ternary Zn-Mg-Al bath has been attributed to the segregation of Al and Mg at grain boundaries which forms Zn/Mg/Al ternary eutectic phase mixture[6]. It has been reported that zinc forms protective corrosion product with Al and Mg[6] and Al rich phase first corrodes, while surrounding ternary eutectic phase mixture remains stable[7,8]. Formation of this protective layer seems to be responsible for better corrosion resistance of Zn-Mg-Al coated sheet. In SEM micrographs (shown in Fig.2) of 0.25% Mg coated sheets, formation of uniform and strong Zn/Mg/Al ternary mixture along the grain boundary was not observed. Though in some isolated regions, ternary mixture of Zn/Mg/Al was observed. This must have led to inferior corrosion resistance properties in 0.25% Mg coated sheets as compared to 0.50 & 0.75% Mg coated sheets. However, in the SEM micrographs (shown in Fig.3&4) of the coated sheets, galvanized in 0.50 & 0.75% Mg bath, it can be clearly seen that there is very prominent and uniform formation of Zn/Mg/Al ternary eutectic phase mixture (composition; Mg : 0.77%, Al : 1.73, Zn : 92.79%) at the grain boundaries due to segregation of Al and Mg at these sites leading to slower anodic dissolution of the Mg-Zn intermetallic phases and formation of protective MgO, which results in enhanced corrosion resistance of Zn-Mg-Al coated sheets [9,10]. Further, corrosion resistance of the Zn-Mg coated sheets was also measured through salt spray test. The results are shown in Table IV. It can be seen that the corrosion resistance of the coated sheets galvanized in Zn-Mg-Al-Sb bath is much superior compared to those galvanized in Zn-Al-Pb, Zn-Al and pure zinc bath.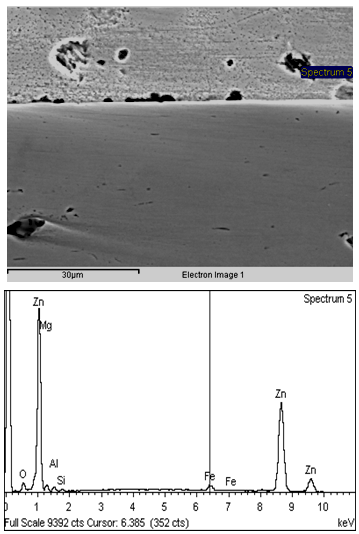 | Figure 2. Microstructure and EDAX analysis of coated sheet galvanized in 0.25%Mg bath |
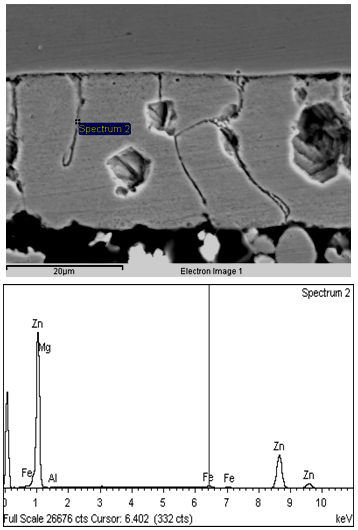 | Figure 3. Microstructure and EDAX analysis of coated sheet galvanized in 0.50%Mg bath |
 | Figure 4. Microstructure and EDAX analysis of coated sheet galvanized in 0.75%Mg bath |
Table 4. Salt spray test results of the coated sheets
 |
| |
|
In case of pure Zn, Zn-0.20Al and Zn-0.20-0.10Pb coated sheets, 100% white rust formation takes place within 6 hrs, 14 hrs and 18 hrs. respectively, while in case of Zn-Mg-Al-Sb coated 100% white rust coverage on the sheet surface happens only after 32 hrs. of exposure. This happens because Zn-Mg coatings inhibit the cathodic reaction and thus improve the corrosion resistance. The usual zinc carbonate hydroxide and zinc oxide observed in Zn-0.20Al and pure Zn coatings are suppressed in the presence of magnesium in the coating, and the whole surface is covered with zinc chloride hydroxide, which is protective for the coating layer.
3.2. Formability property of Zn-Mg Coated Sheets
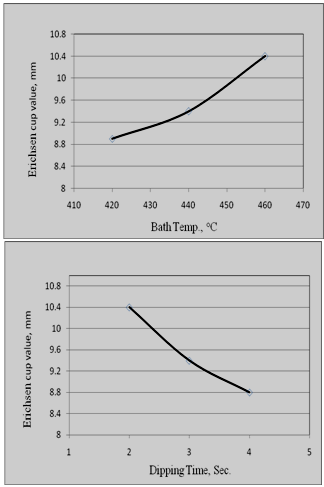 | Figure 5. Influence of bath temp. and dipping time on formability property of Zn-Mg coated sheets |
The formability characteristics of the newly developed coated sheets were quantified in terms of erichsen cup value of the composite. In the simulated GI sheets, erichsen cup value (Ev) of the composite varied in the range of 8.8 mm to 10.4 mm depending on the bath composition and processing conditions of the sheet. Effect of bath temperature and dipping time on the Ev value of the coated sheets has been shown in Fig.5.It can be seen that with increase in dipping time (for a particular bath composition and bath temperature), Ev value decreases slightly. This may be attributed to the fact that with increase in residence time, the thickness of alloy layer (Fe-Zn intermetallics), increases slightly. However, with increase in bath temperature (for a particular bath composition and dipping time), Ev value improves. Generally, formability of the galvanized sheets decreases with increase in bath temp., as increase in the bath temperature is generally associated with enhancement in the kinetics of the formation of Fe-Zn IMCs. However, in this case, due to presence of adequate Si (0.08%) and Al (~0.25%) in the zinc bath, tendency for the formation of Fe-Zn IMC gets significantly reduced (as shown in Fig.6). and this leads to improvement in formability characteristics of the coated sheets. 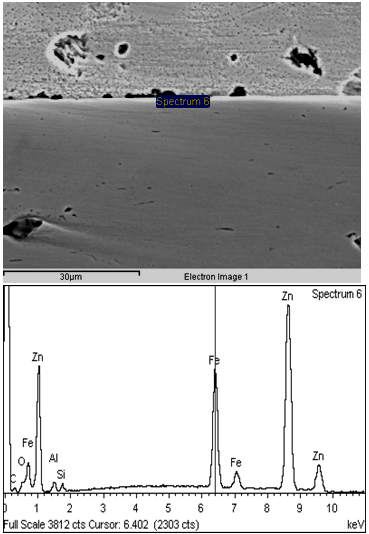 | Figure 6. Microstructure and EDAX analysis of Zn-Mg coated sheet showing presence of Si and Al at the coating –Steel interface |
 | Figure 7. Strain analysis in formed component of (a) Conventional GI sheet (b) Zn-Mg coated sheet |
In order to assess the forming capability of Zn-Mg coated sheets in actual stamping operation, grid marking of coated sheets were done and subsequently these sheets tested in Erichsen cup tester. The coated sheets were deformed till fracturing of the composite. Strain level near the fracture zone was measured through transparent scale. It has been found that newly developed Zn-Mg coated sheets can be safely deformed upto 30% strain. No cracks were visible even at fracturing of the composite. However, in conventional zinc coated sheets, even at 25% strain level cracks can be seen in the coating before the fracturing of the composite (as shown in Fig.7.).
3.3. Assessment of Coating Adherence
Coating adherence of the Zn-Mg coated sheets was assessed through Lock Forming Tester. Most of the sheets manifested excellent coating adherence (as shown in Fig.8), no cracks were observed near the bend portion. However, in some cases, minor cracks were observed.  | Figure 8. Lock Forming Tested Specimen with (a) poor and (b) excellent coating adherence of Zn-Mg coated sheet |
On examining these samples, it has been found that most of these samples were processed at lower bath temperature (420 oC) for higher dipping time (4.0 sec.). Due to lower solubility of Si at this temperature and higher residence time of the strip at this temperature, some formation of Fe-Zn IMC does take place and this leads to poor coating adherence. In some of the samples, poor coating adherence was observed due to entrapment of Sb containing exogenous compound (having composition Sb : 75.38%, Mg : 18.85%, Zn : 5.76%), as shown in Fig.9. This must have happened due to improper mixing of Sb (due to its higher melting point) in the zinc bath.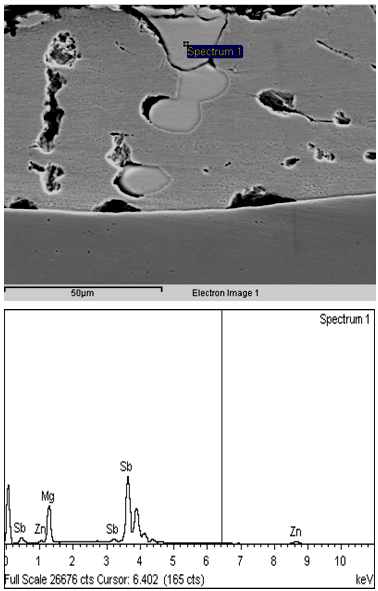 | Figure 9. Microstructure and EDAX analysis of Zn-Mg coated sheet showing entrapment of exogeneous compound in the coating |
4. Conclusions
Based on the work carried out, following conclusions can be drawn :• Addition of Mg (0.50-0.75%) and Sb (~0.08%) in the zinc bath results in substantial improvement in corrosion resistant properties of the zinc coated sheet due to formation of Zn/Al/Mg eutectic mixture at grain boundaries and distribution of Sb within the coating. • Si (~0.08%) addition along with Al (~0.25%) in the bath leads to complete suppression of the formation brittle Fe-Zn intermetallics at the coating-steel interface, thereby resulting in significant improvement in the coating adherence and formability properties of Zn-Mg coatings.• Zn-0.50%Mg-0.25%Al-0.08%Si coated steel sheets with improved corrosion resistance (1/3rd w.r.t conventional Zn coated sheets), formability properties (at par with substrate material) and coating adherence (as per LFQ standard) with bright and smooth appearance have been successfully developed in the laboratory.
ACKNOWLEDGEMENTS
The authors are grateful to the management of Research & Development Centre for Iron & Steel, Ranchi for their constant support and encouragement during the course of work accomplished under this project.
References
| [1] | J. Kawafuku, J. katosh, M. Toyama, H. Nishimoto, K. Ikeda and H. satoh, “Structure and corrosion resistance of Zinc alloy coated steel sheets obtained by continuous vapor deposition apparatus”, Tetsu-to-hagane, 77, 1991, 995-1002. |
| [2] | H. Shindo, K. Nishimura and K. Saito, “Anti-corrosion in atmospheric Exposure of Zn-Mg-Al Hot-dip Galvanized steel sheet”, Proc. Of 4th Int. Conf. on Zinc and Zinc alloy coated steel sheet, Galvatech’98, Tokyo, Japan, 1998, 433-436. |
| [3] | Takao Tsujimura, Atsushi Komatsu, Atsushi Andoh, “Influence of Mg content in coating layer and coating structure on corrosion resistance of Hot-dip Zn-Al-Mg Alloy Coated Sheet, 5th int. Conf. on Zinc and Zinc alloy coated steel sheet, Galvatech’01, Brussels, Belgium, 2001, 145-152. |
| [4] | S.J. Kim, Y. Mizutani, R. Ichino, M. Okido, “Surface morphology and electrochemical property of Mg-Al alloys anodized in alkaline solutions and sealed, 203rd meeting-Paris, France, April, 2003, 244. |
| [5] | Shiwei Li, Bo Gao, Ganfeng Tu Yi Hao, Liang Hu and Shaohua Yin,“Study on the corrosion Mechanism of Zn-5Al-0.5Mg-0.08Si Coating“, Journal of Metallurgy, Volume 2011, 2011, Article ID 917469, 5 pages |
| [6] | R. Hausbrand, M. Rohwerder, M. Stratmann, C. Schowerdt, B. Schumacher, G. Grundmeier, “Model study on the corrosion of Mg containing zinc coatings on the steel sheet”, 5th int. Conf. on Zinc and Zinc alloy coated steel sheet, Galvatech’01, Brussels, Belgium, 2001, 161-167. |
| [7] | T. Prosek, A. Nazarov, U. Bexell, D. Thierry, J. Serak, Corrosion properties of model Zinc-Magnesium alloys, 7th Int. Conf. Zinc and Zinc alloy coated steel sheet, Galvatech’07, Osaka, Japan, 2007, 592-597. |
| [8] | S. Tanaka, K. Hoda, A. Takahashi, Y. Morimoto, M. Kurosaki, H. Shindou, K. Nishimura, M. Suguyama, “The performance of Zn-Al-Mg-Si Hot-Dip Galvanized Steel Sheet”, 5th int. Conf. on Zinc and Zinc alloy coated steel sheet, Galvatech’01, Brussels, Belgium, 2001, 153-160. |
| [9] | S. Schuerz, M. Fleischanderi, G.H. Luckeneder, K. Preis, T. Haunschmied, G. Mori, A.C. Kneissi,“Corrosion bevahior of Zn-Al-Mg Coated steel sheet in sodium chloride-containing environment“, Corrosion Science, Vol. 51, Issue 10, October 2009, 2355-2363. |
| [10] | Monojit Dutta, Arup Kumar Halder, Shiv Brat Singh,“Morphology and properties of hot dip Zn-Mg and Zn-Mg-Al alloy coatings on steel sheet“, Surface and coating Technology, Vol.205, Issue 7, December 2010, 2578-2584. |










 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML
