I. N. Pavlova1, О. S. Travkina1, B. I. Kutepov1, K. R. Ahmed2, A. F. Akhmetov2
1Institute of Petrochemistry and Catalysis RAS, Ufa, 450075, Russia
2Ufa State Petroleum Technological University, Ufa, 450062, Russia
Correspondence to: О. S. Travkina, Institute of Petrochemistry and Catalysis RAS, Ufa, 450075, Russia.
| Email: |  |
Copyright © 2012 Scientific & Academic Publishing. All Rights Reserved.
Abstract
Samples of crystalline aluminosilicate, the granules of which are crystalline aggregates of zeolite X with different degrees of exchange of cations Na+ for cations Ca2+, Mg2+, К+ or Н+ were synthesized. Based on obtained samples, the adsorption of Н2О, СО2, С6Н6 and n-С7Н16 in static and dynamic modes is studied. The dependencies of the amount of adsorbed substance on the type and content of exchangeable cations in zeolite X were found.
Keywords:
Synthesis, Granules, Zeolite Type A, Adsorption
Cite this paper:
I. N. Pavlova, О. S. Travkina, B. I. Kutepov, K. R. Ahmed, A. F. Akhmetov, "Synthesis and Properties Exchange Forms of Granulated Binder-free Zeolite X", International Journal of Materials Engineering, Vol. 2 No. 6, 2012, pp. 80-83. doi: 10.5923/j.ijme.20120206.02.
1. Introduction
Granulated zeolites X in different cationic forms are used in large-tonnage process of adsorption drying and removal of sulfur compounds and СО2, in gas and liquid environments of various composition. Besides, the cationic forms of zeolite X can be used for adsorption separation of mixtures of hydrocarbons. Adsorption cubic capacity and stability when used in cycles of adsorption-desorption of zeolite adsorbents are determined, first of all, by the phase purity and level of crystallinity of zeolite, contained in them, nature and content of exchangeable cations in the zeolite cavities, as well as the parameters of their porous structure.In[1-4] described methods for producing binder-free zeolite NaХ (NaХ-BF), granules of which are crystalline aggregates . Such zeolite granules have higher values of the adsorption characteristics and mechanical strength than the granules with a binder. The presence of cations in the cavities of the porous structure of zeolites causes the following features of zeolites as adsorbents[5]:- the influence of the nature and content of exchangeable cations on the size of the input windows in the cavities;-during exchange of cations Na+ for other cations change of the latter in the cavities is possible, that leads to changes of limiting volume for filling;-the specific interaction of polar molecules with exchangeable cations at low degrees of filling of the adsorption volume.Zeolites X are usually synthesized in the Na-form. Information in literature about obtaining other cationic forms are given only for highly dispersed and granulated zeolite X with a binder [6,7]. There is no such information for binder-free zeolite (BF-zeolite). We can assume that the basic laws of the cationic exchange for binder-free zeolite are the same. At the same time, granules of binder-free zeolite are crystalline aggregates, so they may have specific features of behavior usual for exchange process. There is also no information about influence of type and content of exchangeable cations in the BF-zeolite X on its ability to adsorb substances whose molecules differ in size and structure. According to above-mentioned, present research covers the synthesis of Н+, К+, Ca2+ or Mg2+- forms of BF-zeolite X and the adsorption of molecules of Н2О, СО2, С6Н6 и н-С7Н16 by them in static and dynamic modes.
2. Experimental Part
Н+, К+ , Ca2+ or Mg2+- forms of BF-zeolite X with a diameter of 1.6 mm (HNaХ-BF KNaХ-BF, СaNaХ-BF and MgNaХ-BF) were synthesized from its Na-form using ion exchange in solutions of the respective chlorides. Ion exchange experiments were carried out at 700℃, with initial concentration of salt in a solution of 70 g /l ( the ratio of the excess of second exchangeable cation to sodium), at a solution volume-to-zeolite mass ratio of 4 to 1 in an isothermal reactor of periodical operation during 2 hours with stirring. The degree of exchange of Na+ (αNa) for other cations regulated by the amount of exchanges (from 1 to 4) without intermediate heat treatment. HNa-form of BF-zeolite X (HNaX-BF) was obtained from BF- NH4NaX using heat treatment after the last exchange at 450℃ during 4 hours in the open air. As a comparison with these results, we also obtained exchangeable forms of highly dispersed zeolite NaX with a particle size of 1.0 - 8.0 microns.The chemical compositions of liquid and solid phases were analyzed using gravimetric method, as well as methods of complexometric titration and flame photometry[8].The phase composition of the zeolites were determined using X-ray analysis on an automatic diffractometer PHILIPS PW 1800. To determine used the method of Debye - Scherrer (powder method). Analysis conditions: theta/2 theta -scanning; rotation of holder-one turn sec-1, anode material-copper, range -5-55о/2 theta; step - 0.05°; exposure step - 2 seconds, anode voltage and current - 40 kW and 30 mA, respectively. Radiographs were identified by the known diffraction data[9].To determine the parameters of porous structure ( pore's size and distribution radius, specific surface area) used the low-temperature nitrogen adsorption measured by static vacuum volumetric automated installation "Sorptomatic- 1900" ("Fisons")[10-12], and mercury porosimetry using «Porosimeter-2000"[13].To determine the equilibrium adsorption cubic capacities (cm3/g) for Н2О - А(Н2О), С6Н6 - А(С6Н6) and n-С7Н16 - А(n-С7Н16) of obtained adsorbents used the excicatory method, which is widespread in industry.[14]Measurements were made at 20℃. To determine A (Н2О) in excicator added an aqueous solution of sulfuric acid, which provides P/Ps = 0,7, and pure benzine and heptane to determine A (С6Н6) and A (n-С7Н16) respectively. A (СО2) was measured at concentrations of СО2 in a binary mixture with helium, equal to 70.0 and 0.03% vol. During the experiment samples were removed from the excicator and weighed. The experiment was stopped when a constant sample weight had been obtained. To assess the impact of the replacement of the cations Na+ by other cations on the adsorption cubic capacity of granules of BF-zeolite X in the dynamic mode was measured its adsorption cubic capacity for Н2О - D (Н2О), СО2 - D (СО2), С6Н6 - D (С6Н6) and n-С7Н16 - D (n-С7Н16) from mixtures with air in a flow adsorber at atmospheric pressure, temperature 20-25°C and the amount of the loaded adsorbent up to 150 сm3. The rate of steam or air-gas flow was 4,0 ± 0,25 dm3/min for a mixture with С6Н6 and n-С7Н16 at vapor concentrations of 12-15 mg/ dm3, and 1,0 ± 0,25 dm3/min for a mixture of Н2О and СО2 at a concentration of 13-15mg/dm3 and 200 mg/dm3, respectively. The adsorption experiment was stopped after reaching "the skip" concentration corresponding to-70℃ for Н2О and -60℃ for С6Н6 and n-С7Н16. In the case of СО2 experiment was stopped when it comes out of the adsorber.
3. Results and Discussion
Figure 1 shows the results of studying the influence of the nature of exchangeable cation and the number of treatments with Na during exchange for cations Н+, К+, Ca2+ or Mg2+ in the zeolite NaХ-BF. To compare results here stated data of similar exchanges in the samples of highly dispersed zeolite NaХ.Presented results show that in order to achieve maximum αNa in zeolite NaХ - BF three exchange treatments are required. Any further increasing in their number has no significant impact. 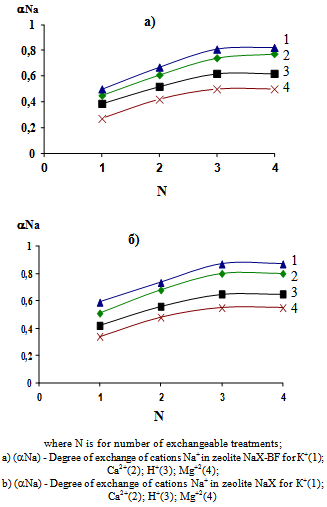 | Figure 1. Figure 1. Influence of the nature of exchangeable cation and number of treatments on the degree of exchange in the granular (a) and highly dispersed (b) zeolites of type X |
Comparison of values of αNa in granular and highly dispersed samples with the same number of treatments, showed that the granular exchange proceeds more slowly, apparently due to diffusion limitations. The maximum values of αNa during exchange for cations Н+, К+, Ca2+ or Mg2+ are 0.64, 0.82; 0.79 and 0.52, respectively. The discrepancy between the values is determined by difference of their own size and energy of hydration of above-mentioned ions[6, 7]. For highly dispersed zeolite NaХ αNa is 0.66, 0.87, 0.83, 0.56, respectively (Fig. 1).X-ray analysis shows that degree of crystallinity of the highly dispersed zeolite X before exchange is close to 100.0%, and for the NaХ-BF it makes 90,0-94,0%. After an exchange of Nа+ cations for the above-mentioned cations, these values are almost unchanged. The values of the mechanical crush strength of granules (butt-end crush) during of the exchange does not change and make 2.0-2.2 kg/mm2. Taking into account the fact that the characteristics of granulated zeolite's pore structure have a significant impact on the amount of material adsorbed in the dynamic mode, these characteristics of samples of zeolite NaХ-BF were measured before and after the exchange treatments. The results are shown in Table 1. Results of determination of A (Н2О), А(н-С7Н16) and А(С6Н6) for zeolite X-BF in the above-mentioned cationic forms as well as similar characteristics of the samples of powdered zeolite X for comparison are shown in Tables 2-4.| Table 1. Parameters of the porous structure of cationic forms of zeolite X-BF |
| | Cationic form of zeolite | Vpor, cm3/g | Roaverage, Å | Sspecific, m2/g | Sspecific, m2/g nitrogen | | mercury | | NaХ-BF | 0,24 | 963 | 3,9 | 364 | | 0,64НNaХ-BF | 0,35 | 1250 | 7,9 | 370 | | 0,82КNaХ-BF | 0,30 | 1230 | 7,5 | 316 | | 0,79СаNaХ-BF | 0,29 | 1258 | 5,8 | 403 | | 0,52MgNaХ-BF | 0,28 | 1222 | 5,4 | 396 |
|
|
Data from Table 1 shows that the parameters of the porous structure of granules after the exchange remain unchanged.| Table 2. A (Н2О) of zeolites X in different cationic forms |
| | Cationic form of zeolite | А(Н2О)cm3/g | Cationic form of zeolite | А(Н2О) cm3/g | | NaХ-BF | 0,27 | NaХ | 0,30 | | 0,64НNaХ-BF | 0,22 | 0,66НNaХ | 0,25 | | 0,82КNaХ-BF | 0,26 | 0,87КNaХ | 0,29 | | 0,79СаNaХ-BF | 0,27 | 0,83СаNaХ | 0,29 | | 0,52MgNaХ-BF | 0,28 | 0,56MgNaХ | 0,32 |
|
|
| Table 3. А(n-С7Н16) of zeolites X in different cationic forms |
| | Cationic form of zeolite | А(n-С7Н16) cm3/g | Cationic form of zeolite | А(n-С7Н16) cm3/g | | NaХ-BF | 0,28 | NaХ | 0,30 | | 0,64НNaХ-BF | 0,23 | 0,66НNaХ | 0,27 | | 0,82КNaХ-BF | 0,27 | 0,87КNaХ | 0,29 | | 0,79СаNaХ-BF | 0,29 | 0,83СаNaХ | 0,30 | | 0,52MgNaХ-BF | 0,28 | 0,56MgNaХ | 0,31 |
|
|
| Table 4. А(С6Н6) of zeolites X in different cationic forms |
| | Cationic form of zeolite | А(С6Н6) cm3/g | Cationic form of zeolite | А(С6Н6) cm3/g | | NaХ-BF | 0,26 | NaХ | 0,30 | | 0,64НNaХ-BF | 0,22 | 0,66НNaХ | 0,25 | | 0,82КNaХ-BF | 0,26 | 0,87КNaХ | 0.28 | | 0,79СаNaХ-BF | 0,26 | 0,83СаNaХ | 0,29 | | 0,52MgNaХ-BF | 0,27 | 0,55MgNaХ | 0,30 |
|
|
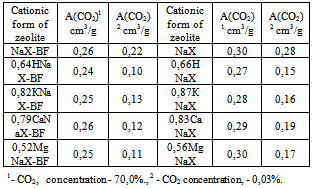
It is obvious that equilibrium adsorptive cubic capacities of granular samples is 10,0-15,0% less than of powdery samples. The main reason of smaller adsorptive cubic capacity of granules is that they correspond to an aggregate of nanodimensional crystals and part of its intracrystalline space can be inaccessible for molecules of adsorbate.The results, represented in tables 2-4, show that during transition from NaX- BF to KNaX-BF, CaNaX-BF or MgNaX-BF with maximum degrees of exchange, there were no considerable changes in the values of А(Н2О), А(С6Н6) and А(н-С7Н16).Table 5 represents the results of the research of СО2 adsorption on the zeolite X in different cationic forms. It is clear that when the СО2 concentration is equal to 70,0%, the values of maximum CO2 adsorptive cubic capacities for all cationic forms of zeolites X are also similar (table 5).Table 5. А (СО2) of zeolites X in different cationic forms
 |
| |
|
The obtained results are explained by the fact that in the researched conditions volumetric filling of microcellular intracrystalline space of zeolite X-BF takes place. In this case А(Н2О), А(С6Н6), А(n-С7Н16) and А(СО2) (СО2 concentration = 70,0%.) are mainly defined by its volume and by sizes of entrance windows in cavity. In transition from one cationic form, studied in this work, to another the indicated characteristics in zeolite X practically do not change.The nature and content of the cation in zeolite Х-BF become apparent in case of small degrees of filling. In the present work such results were obtained for СО2. From the data of table 5 it is clear that NaX-BF, for which the lower surface acidity is characteristic, adsorb CO2 when its concentration is equal to 0,03%, that is 1,5-2 times more than other exchange forms of the same zeolites.| Table 6. А (СО2) of zeolites X in different cationic forms |
| | Cationic form of zeolite | D(Н2О) mg/cm3 | D(n-С7Н16) mg/cm3 | D(С6Н6) mg/cm3 | D(CO2) mg/cm3 | | NaХ-BF | 159 | 90 | 88 | 55 | | 0,64НNaХ-BF | 109 | 65 | 62 | 24 | | 0,82КNaХ-BF | 139 | 89 | 87 | 54 | | 0,79CaNaХ-BF | 143 | 80 | 88 | 49 | | 0,52MgNaХ-BF | 138 | 82 | 80 | 48 |
|
|
Table 6 contains the results of the research concerning the influence of type and content of exchange cations in zeolite Х-BF on its adsorptive cubic capacities (mg/sm3) of H2O – D(H2O) , n-C7H16 – D(n-C7H16), C6H6 - D(C6H6) and CO2 – D(CO2) in running adsorber.It is obvious that in flow reactor with high speeds of gas stream the differences in values of D(Н2О), D(С6Н6), D(n-С7Н16) and D(CO2) for all cationic forms of zeolites X do not exceed experimental error.Other results were obtained while studying adsorption on decationed form of zeolite X-BF with the degree of exchange of cations Na+ for Н+ equal to 0,6. The data in tables 2-4 show that adsorptive cubic capacities of НNaХ-BF, especially in flow regime, are considerably reduce. The maximum decrease typical for CO2 because decating results in the increase of zeolite surface acidity. We can also suppose that removal from the intracrystalline space of cations, that are able to interact with the molecules of adsorbate, reduce the diffusion rate of the latter in the cavity of zeolite.
4. Conclusions
Ion exchange of cations Na+ for cations Н+, К+, Ca2+ or Mg2+ in granular zeolite binder-free NaХ was studied. This study showed that degree of exchange after first processing and final degrees of exchange are 10,0- 15,0% lower than exchange in fine-grained samples because of lower cations’ diffusion rate and inaccessibility of the part of intracrystalline space in granules, which correspond to integrated crystalline aggregates. It was found out that in granules the maximum values of the degrees of exchange of Na+ for Н+, К+ , Ca2+ or Mg2+ are different: 0,64; 0,82; 0,79; 0,52 accordingly. After exchange high degree of crystallinity and parameters of porous structure of granules are invariable.It was found out that granular zeolite Х without binding material in different cationic forms has maximum adsorptive cubic capacities of Н2О, СО2, С6Н6 and n-С7Н16 that are 10,0-15,0% lower than maximum adsorptive cubic capacities of fine-grained samples because of inaccessibility of the part of intracrystalline space in granules. The research showed that in zeolite Х-BF during the transition from Na-form to K, Ca or Mg-form with maximum degrees of exchange and in condition of 20ºC and high concentrations of adsorbates there is no significant changes of equilibrium adsorptive cubic capacities of Н2О, СО2, С6Н6 and n-С7Н16. If the СО2 concentration is 0,03% - because of its minimal acidity 1,5-2 times more СО2 adsorbs on Na-form of the zeolites X than on other exchange forms of the same zeolites. It was also found out that production of decationed form of zeolite Х-BF results in reduction of its adsorption property. For HNa-forms with the degrees of exchange of 0,6 adsorptive cubic capacities of Н2О and СО2 in flow reactor reduce for 1,5 and 2,0 times accordingly.
References
| [1] | Pat. RUS № 2203224 Method of obtainment of synthetic granular faujasite of high phase purity. Glukhov V.A., Glukhov A.V. |
| [2] | Pat. RUS № 2283278 Method of obtainment of granular zeolite adsorbent of structure A and X of high phase purity. Rakhimov Kh.Kh., Pavlov M.L., Kutepov B.I., Makhamatkhanov R.A. and others |
| [3] | Pat. RUS № 2322391 Method of obtainment of synthetic granular zeolite of X type. Glukhov V.A., Zelenov L.E., Zelenov A.V. |
| [4] | Kutepov B.I., Pavlov M.L., Pavlovа I.N., Travkina O.S., Basimova R.A. «Syntheses of high-performance A and X type binder-free zeolites from kaolin», Petrochemistry, vol.49, no.1, pp.39-442, 2009 |
| [5] | Zhdanov S.P., Khvoschev S.S., Samulevich N.N. Synthetic zeolites, 264p., 1981 |
| [6] | Breck D. Zeolite molecular sieves, 781p., 1976 |
| [7] | Tolmachev A.M. The study of zeolites as selective ion exchangers for division of mixes of substances similar in qualities and isotopes. Modern problems of physical chemistry., vol.10, pp.134-190, 1978 |
| [8] | Kreschkov A.P., Yaroslavtsev A.A. Course of analytical chemistry., 471p., 1975 |
| [9] | Treacy M.M.J., Higgins J.B. Collection of Simulated XRD Powder Patterns for Zeolites. Amsterdam - London - New York - Oxford - Paris - Shannon – Tokyo, Elsevier, 586 p., 2001 |
| [10] | Branawer S. Adsorption of gases and steams., Т. I., 781p., 1948 |
| [11] | Greg S., Sing K. Adsorption. Specific surface. Porosity., 310p., 1984. |
| [12] | V.B. Fenelonov, L.G. Okkel, N.S. Slydkina, T.M. Malygina Devices and technics of experiment., no.4., pp.133-136, 1997. |
| [13] | Plachenov T.G., Kolosentsev S.D. Porometry., 175p., 1988 |
| [14] | Keltsev N.V. Basis of adsorptive technics., 592p., 1984 |


 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-Text HTML
Full-Text HTML