-
Paper Information
- Next Paper
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
International Journal of Materials Engineering
2012; 2(4): 38-42
doi: 10.5923/j.ijme.20120204.02
Effect of Anodization on the corrosion behavior of Aluminium Alloy in HCl acid and NaOH
Madakson P. B , Malik I. A , Laminu S. K , Bashir I. G.
Department of Mechanical Engineering, Ahmadu Bello University, Zaria, Nigeria
Correspondence to: Malik I. A , Department of Mechanical Engineering, Ahmadu Bello University, Zaria, Nigeria.
| Email: |  |
Copyright © 2012 Scientific & Academic Publishing. All Rights Reserved.
The effect of anodic oxidation on the corrosion behaviour of Aluminium alloy ‘7075’ in two different media was investigated. The weight loss technique was used to determine the corrosion rate in 1.0M HCl and 1.0M NaOH solutions. These specimens were allowed to stay in the solutions for 504 hours with an interval of 72 hours weight loss measurement. The results shows that in both media, the normal corrosion rate profile of an initial steep rise in corrosion followed by subsequent fall was observed for both anodized and unanodized specimens but with severe attack on those specimens in 1.0M HCl. The anodized Al-Alloys in both solutions have lesser corrosion rate as compared to the unanodized Alloys. It was concluded that, Aluminium Alloy ‘7075’ corrodes faster in 1.0M HCl than in 1.0M NaOH solution and that anodizing helps to reduce the corrosion rate of Al- Alloy in both media.
Keywords: Aluminium Alloy, Corrosion, Corrosion Rate, Weight Loss
1 Introduction
- New developments in the aircraft field are intimately related to the development and use of materials which are stronger, lighter, more corrosion resistant and more temperature resistant. In the area of structural efficiency, evolutionary changes have taken place which had provided significant weight savings in airframe structures, and it is in the area that major increases in efficiency in the future are possible.[1].Although metals are very important engineering materials, but the serious setback to their mechanical properties is corrosion. The effect of unchecked corrosion therefore is not limited to the state of corroding utility itself but also influences deeply man and his economic and social welfare.Corrosion has many serious economic, health, safety, technological, and cultural consequences to our society.In the broad sense, corrosion may be defined as “the destruction of a material by chemical, electrochemical, or metallurgical interaction between the environment and the material”[2]. Generally it is slow but persistent in character. In some cases, the corrosion products exist as a thin adherent film which merely stains or tarnishes the metal and may act as a retardant to further corrosive action. In other cases, the products of corrosion are bulky and porous in character, offering no protection. One of the most serious problems of industry, corrosion causes damage in the billions of dollarseach year. The science of corrosion prevention and control is highly complex, exacerbated by the fact that corrosion takes many different forms and is affected by numerous outside factors[1]. Corrosion professionals must understand the effects of environmental conditions such as soil resistivity, humidity and exposure to salt water on various types of materials; the type of product to be processed, handled, or transported, required lifetime of the structure or component, appropriate mitigation methods and other considerations before determining the specific corrosion problem and specifying an effective solution. Corrosion may take place by direct reaction between the metal and the solution in contact with it, or the corrosion reaction may separate into anodic and cathodic parts which may take place at areas separated from each other by finite distances. If the reaction takes place without separation, it is called direct chemical corrosion and if the two parts are separated it is called electrolytic corrosion. Whether it is direct chemical or electrolytic, the course of the reaction must be in conformity with the known laws of electrochemistry andthermodynamics[3]. Causes of CorrosionThe basic cause of corrosion is the instability of metals in their refined forms. The metals tend to revert to their natural states through the processes of corrosion. One of the most important factors influencing corrosion is the difference in electrical potential of dissimilar metals when coupled together and immersed in an electrolyte. This potential is due to the chemical natures of the anodic and cathodic regions. Agitation acts to increase the corrosion rate by bringing fresh corroding solution into contact with the metal. Differences in potential from point to point on a single metal surface cause corrosion known as local action and may be due to impurities on the surface or differences in surface structure or environment. Other factors-such as the presence of other ions in solution, the temperature of the solution and the existence of stray electric current-may materially affect the corrosion rate[2]. All forms of corrosion, with the exception of some types of high-temperature corrosion, occur through the action of the electrochemical cell. The elements that are common to all corrosion cells are an anode where oxidation and metal loss occur, a cathode where reduction and protective effects occur, metallic and electrolytic paths between the anode and cathode through which electronic and ionic current flows, and a potential difference that drives the cell. The driving potential may be the result of differences between the characteristics of dissimilar metals, surface conditions, and the environment, including chemical concentrations. There are specific mechanisms that cause each type of attack, different ways of measuring and predicting them, and various methods that can be used to control corrosion in each of its forms[4].Corrosion PreventionAs is true of the various forms of corrosion, there are many different methods of corrosion prevention and control. Each offers its own complexities and purposes. In general, the approach to control most corrosion is to understand the corrosion mechanism involved and remove one or more of the elements of the corrosion cell; for example, by electrically separating the anode and cathode from each other or from the electrolytic environment by reducing the driving potential. The most commonly used corrosion control methods include materials selection and design, using corrosion-resistant alloys, protective coatings; CP; use of special heat treatment ; corrosion inhibitors. All of these methods are appropriate for controlling corrosion in certain situations and not for others. They are often used together to solve a particular corrosion problem (for example, protective coatings and CP are a common and effectivecombination)[4].Alloy 7075 is from the series of alloy in which itssusceptibility to stress corrosion cracking had been minimized by the addition of chromium and proper heat treatment. They are used in applications requiring high strength and good corrosion resistance, such as aircraft structural parts[5].The high corrosion resistance of aluminium is due to the self-protecting, thin, invisible oxide film that forms immediately on exposing surfaces to the atmosphere. This film protects the metal from further corrosion. If oxide film is removed in many environments, a new film will form immediately and the metal remains fully protected. Alloy 7075 has been thoroughly evaluated for corrosion resistance of atmospheric weathering, stress-corrosion cracking and exfoliation in all currently available tempers. These values have been used as a standard for comparison in the development of more recent high strength aerospace alloys. The corrosion resistance of this alloy can be improved by cladding (aclad) or anodizing. AnodizationAnodizing of Aluminium is a well known electrochemical surface treatment during which an anodic oxide layer is formed on an Aluminium anode. Different electrolytes are commonly used, leading to the formation of a porous oxide with pore diameters and barrier layers generally in the range of 10-30 nm whereas total film exceeding 100µm can be obtained[6].The electrolytes that can be used include Chromic acid, Sulphuric acid, Oxalic acid, Borates, citrate and carbonates while employing either alternating or direct current[7]. The part to be anodized is immersed in a suitable electrolytic solution with an insoluble conductor, current is then passed through the conductor to the part to be anodized. The parts to be anodized are usually connected to the positive pole and the insoluble conductor to the negative pole of the electrical source. The inert conductor can either be Aluminium or lead. When current is supplied, oxygen gas will be formed at the anode and Hydrogen gas at the cathode but thee oxygen formed is not liberated but goes into reaction with Aluminium forming a passivating film of Aluminium oxide Al2O3 . As the process continue, the thickness and the rate of growth of the passivating film increases with time[7].In order to render the coating impermeable and non -absorptive to chemicals and other solutions, sealing is carried out in which the alumina produced is converted to hydrated alumina in accordance with reactionAl2O3 + nH2O → Al2O3.nH2OThe amount of Al2O3 formed during anodizing is directly proportional to the current density and time while the growth of the film depends on the chemical composition,concentration of the anodizing electrolyte and the anodizing conditions[8]. Some of the electrolytes have little or no solvent action on the oxide coating so that very thining films usually known as barrier layer type coatings are formed, the thickmess of which is solely dependent on applied voltage. These type of coatings are typically produced in solutions of borate, boric acid or titrate. Sulphuric acid is an example of electrolytes that as solvent action on the formed coating, porous film are also formed as the oxidation process continues leading to the formation of relatively thick film.[9]
2. Experimental Techniques
- Apparatus and MaterialsSpecimen were made from cast Aluminium alloy (equivalent to 7075: Zn ≤ 5.5 wt. %; Cu ≤ 2.5 wt. % Mg ≤ 1.5 wt. % and Al ≥ 90.5 wt. %). The rods were machined to 20mm diameter and 18mm in length (cylindrical in shape). The cylindrical samples were made smooth by thoroughly grinding on emery abrasive papers. The corrosive media consists of 1.0M solution of each of HCl(aq) and NaOH(aq) .Other materials include beakers, ethanol, distilled water,mettler electronic weighing balance, and abrasive emery grinding papers.Materials and conditions of anodizing1. Variable transformer: Model No: (type V6H-PSV), input voltage 240V, 50Hz. Output voltage 0-27V (3Amp).This was connected to the main A.C source, 50Hz, 220V. It stepped down and converted the A.C voltage to D.C voltage (variable supply depending on the desired working voltage).2. A battery charge (Rectifier): Produced by DYNAMIC SWISS ACCU company limited, London with serial number 7105/536. This rectifier helped to stabilize the voltage and reveal the operating current with the help of its dial pointer.3. Electrolyte Bath: Comprises of one litre capacity pyrex beaker, a plank of dimension 90mm by 60mm for rigidity of the circuit electrolyte during the process.4. Electrodes: This comprises of a thin aluminium sheet 0.50mm thick with dimension of 80mm by 30mm.5. Electrolyte: The electrolyte used is tetraoxosulphate VI acid 98.5% purity.Six cylindrical samples were kept aside for anodizing. The anodizing conditions are described below: Anodizing voltage = 12 voltsAnodizing temperature = 30℃ AmbientElectrode dimension = 80mm by 30mmAnodic current = 3 AmpElectrolyte concentration = 3.67MAnodizing time = 30 minutesExperimental procedureAll the test coupons were cleaned, weighted and stored in a dessicator. The weighted coupons were immersedcompletely into 500ml different beakers containing the corrosive media, weight losses of the coupons were taken at intervals of 72 hrs (3 days) over a period of 504 hrs (21 days). Before each coupon was weighed, the surface was scrubbed with brush in distilled water and rinsed in ethanol in order to remove corrosion product and then air-dried. The weight loss was determined as the difference between the initial weight of the specimen prior to immersion and its weight after removal of corrosion product. Each reading was recorded to the nearest 0.0001g on a mettler electronic weighing balance.Calculation of the corrosion rate is in mm/year. Using the formula,
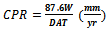 | (1) |
3. Results and Discussion
- ResultsThe corrosion results and all measurements carried out are as shown in Tables1 to 4 and Figures 1 to 4.Discussion of resultsFrom figure 1 and 2, it can be observed that the corrosion rate of Aluminium alloy is greater in 1.0M HCl solution than in 1.0M NaOH solution. This implies that Aluminium Alloy 7075 corrode faster in acidic medium than in basic medium at particular concentration. Both figures show the normal corrosion profile of most passivating metals subjected to corrosive environments. Aluminium is known to belong to the group of these metals. The trends show the initial steep rise in corrosion rate corresponding to the active region, until a peak value is reached, after which due to adherence of the formed oxides on the metal surface, the corrosion rate progressively declines as a result of passivity. Slight increase in the corrosion rate after decrease was due to trans-passive region.From figure 3, it can be seen that there is reduction in the corrosion rate, it can also be seen that there is reduction in the weight loss of the anodized Al-Alloy 7075 in 1.0M HCl with respect to exposure time as compared to the unanodized Alloy. This means that the process of anodizing reduces the corrosion rate of the Alloy in acidic medium.From figure 4, it can be seen that there is reduction in the corrosion rate of the anodized AL-Alloy 7075 in 1.0M NaOH with respect to exposure time. This also signifies that anodizing helps to reduce the corrosion rate in basic medium (1.0M NaOH).
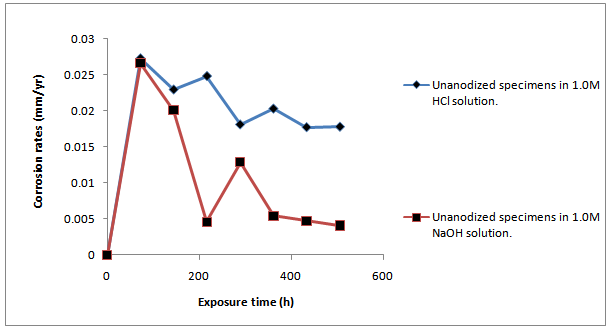 | Figure 1. Graph of Corrosion Rate of Unanodized Al-Alloy ‘7075’ in 1.0m Hcl and 1.0m Naoh Solution Against the Exposure Time |
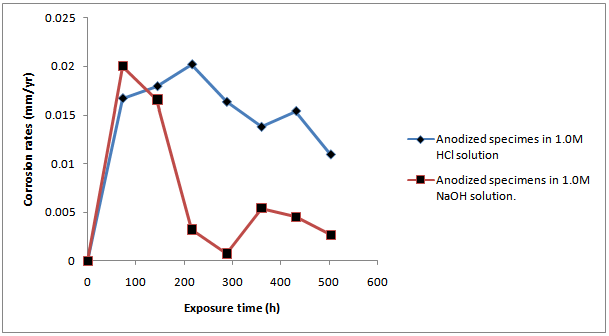 | Figure 2. Graph of Corrosion Rate of Anodized Al-Alloy ‘7075’ In 1.0m Hcl and 1.0m Naoh Solution Against the Exposure Time |
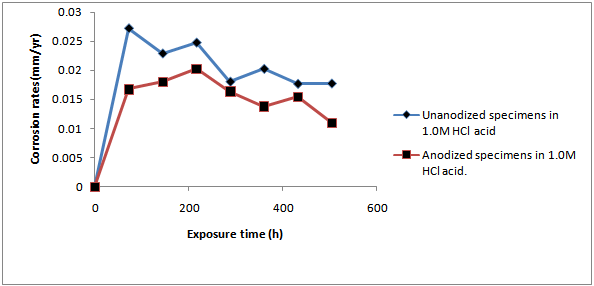 | Figure 3. Graph of Corrosion Rate Ofanodized and Unanodized Alalloy‘7075’Against Exposure Time in 1.0m Hcl Acid |
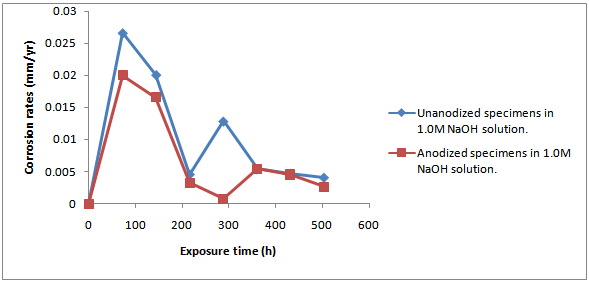 | Figure 4. Graph Of Corrosion Rate of Anodized and Unanodized Al-Alloy ‘7075’ Against Exposure Time in 1.0m Naoh Solution |
4. Conclusions
- After careful analysis of the results obtained, the following were noted: The Aluminium Alloy, both anodized and unanodized has higher corrosion rate in 1.0M HCl than in 1.0M NaOH solutions.Process of anodizing reduced the rate of corrosion of the Alloy in both the acidic and basic medium.The presence of Cl- serves to improve the destruction of the passive films at localized regions.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-Text HTML
Full-Text HTML