-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
International Journal of Materials and Chemistry
p-ISSN: 2166-5346 e-ISSN: 2166-5354
2026; 16(1): 1-5
doi:10.5923/j.ijmc.20261601.01
Received: Dec. 23, 2025; Accepted: Jan. 19, 2026; Published: Jan. 22, 2026

Coordination Compounds of Barium Nitrate Strontium with Formamid and Carbamid
Z. K. Jumanazarova , A. P. Bektursinova , G. A. Ilalova , O. N. Matkurbanova
Karakalpak State University, Uzbekistan
Copyright © 2026 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Mixed-ligand coordination compounds of barium and strontium nitrates with formamide and carbamide have been synthesized. The composition, individuality, and coordination methods of formamide, carbamide and nitrate fragment molecules of coordination compounds were established, and the thermal behavior of the obtained complexes was studied.
Keywords: Physicochemical analysis methods, IR absorption spectra, X-ray phase analysis, Coordination compounds, Synthesis, Composition, Thermal behavior
Cite this paper: Z. K. Jumanazarova , A. P. Bektursinova , G. A. Ilalova , O. N. Matkurbanova , Coordination Compounds of Barium Nitrate Strontium with Formamid and Carbamid, International Journal of Materials and Chemistry, Vol. 16 No. 1, 2026, pp. 1-5. doi: 10.5923/j.ijmc.20261601.01.
Article Outline
1. Introduction
- Developing the synthesis of new chemical compounds with effective properties for use in agriculture is one of the urgent tasks of modern chemistry. mixed-ligand complex compounds of metals, possessing a number of specific properties, have found wide application in various sectors of the national economy. The use of substances containing donor atoms of aliphatic and carboxylic acid amides, in particular formamide (FA), carbamide (K), as ligands contributes to the formation of coordination compounds containing macroelements [1].Numerous works by authors such as Yunusov D.Kh., Mukumova G.Zh., Kuzmin N.E., Palkin K.K., Parpiev N.A., Azizov T.A., Azizov O.T., Suleymanova G.G., Azizzhanov Kh.M., Khasanov Sh.B. and others have been devoted to the synthesis and study of mixed-amide complex compounds of metal nitrates. There is no data in the literature on mixed-ligand coordination compounds of barium nitrate. Our goal is to synthesize the coordination compounds of barium and strontium nitrates with formamide and carbamide. Establishing the composition, individuality, and coordination methods of formamide, carbamide, and nitrate fragment molecules. Study of the thermal behavior of synthesized compounds.
2. Research Objects and Methods
- For the synthesis of coordination compounds, we chose a mechanochemical method, as it does not require scarce organic solvents and allows for the synthesis of complexes of various compositions with a high yield in a short time. The synthesis was carried out according to the method [2].The analysis of the synthesized compounds for magnesium and calcium content was carried out according to [3]. Nitrogen was determined by the Dumas method [4], carbon and hydrogen with combustion in oxygen current (Table 1). To establish the individuality of the synthesized compounds, diffractograms were taken on a DRON-2.0 device with a Cu-anticathode [5]. IR absorption spectra were recorded in the region of 400-4000 cm-1 on the AVATAP-360 spectrometer of the "Nicolet" company. Thermal analysis was carried out on a derivatograph of the F.Paulik, J.Paulik, L.Erdey system [6] at a rate of 9 deg/min and a sample of 0.102-0.143 g. at the sensitivity of galvanometers T-900, TG-200, DTA, DTG-1/10. The recording was carried out under atmospheric conditions. The holder was a 10 mm diameter platinum crucible without a lid. Al2O3 was used as a standard. A complex compound of the composition Ba(NO3)2·2HCONH2·2CO(NH2)2 was synthesized by mixing 1.3056 g (0.005 mol) of barium nitrate with 0.4504 g (0.01 mol) of formamide and 0.6008 g (0.01 mol) of carbamide in an agate mortar at room temperature for 3 hours. The product yield is 92.01%.During the synthesis of a complex compound with the composition Sr(NO3)2·2HCONH2·2CO(NH2)2, 1.065 g (0.005 mol) of strontium nitrate was ground with 0.4504 g (0.01 mol) of formamide and 0.6008 g (0.01 mol) of carbamide in an agate mortar at room temperature for 3 hours. The complex yield is 98.94%.
3. Results and Their Discussion
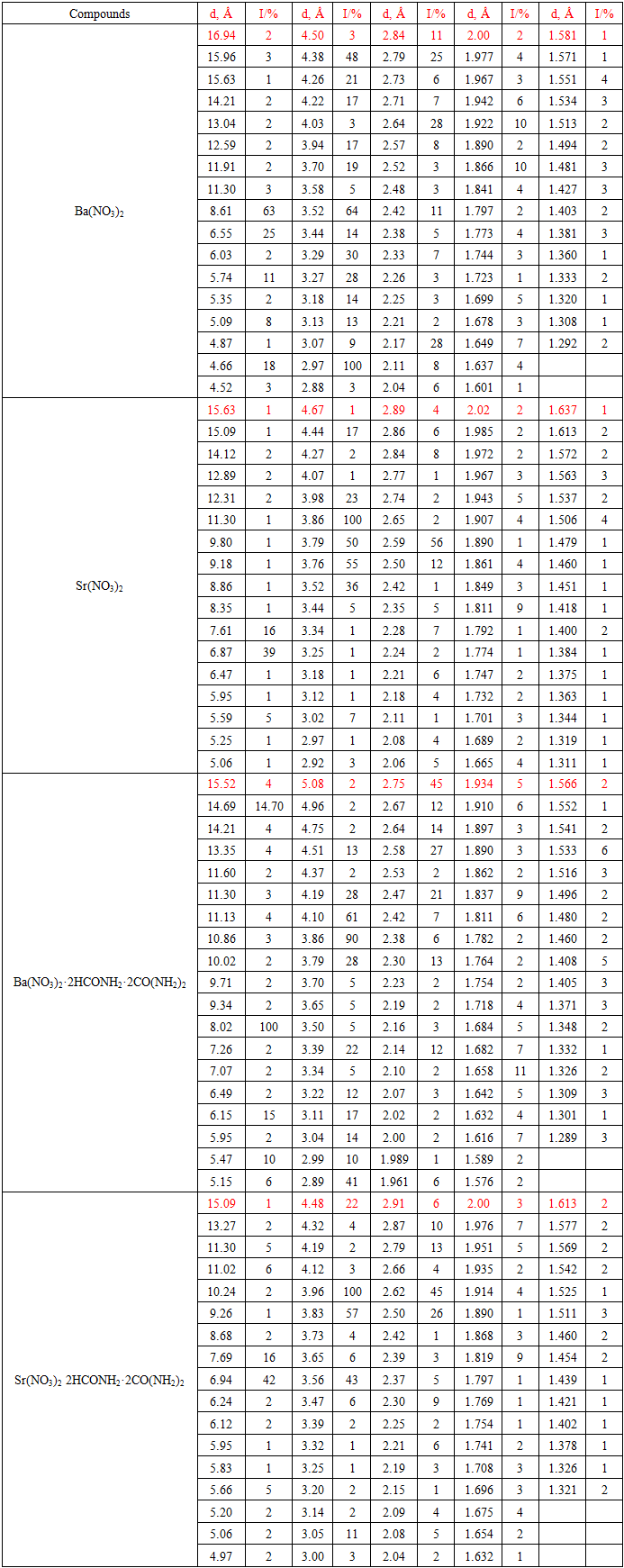
- Comparison of the interplanar distances and relative intensities of barium and strontium nitrate, formamide, carbamide and coordination compounds of the compositions [Ba(NO3)2·2HCONH2·2CO(NH2)2], [Sr(NO3)2·2HCONH2·2CO(NH2)2] showed that the new coordination compounds differ significantly from each other and from similar initial compounds. Consequently, the synthesized barium and strontium nitrate complexes have individual crystal lattices (Table 1).
|
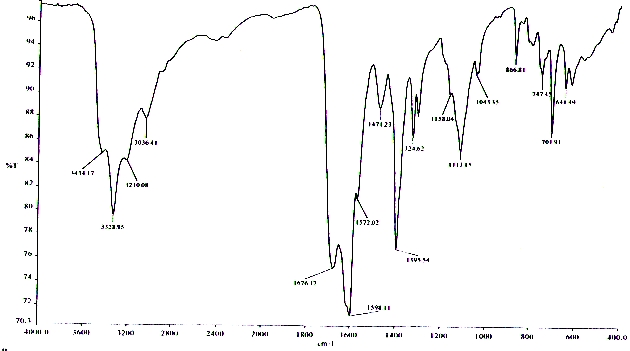 | Figure 1. IR absorption spectrum of the mixed amide complex compound of barium nitrate with formamide and carbamide - Ba(NO3)2·2HCONH2·2CO(NH2)2 |
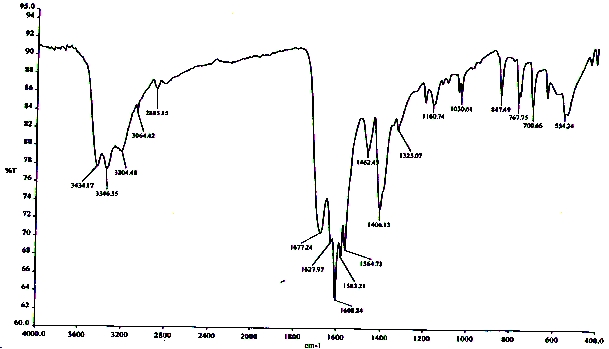 | Figure 2. IR absorption spectrum of the different amide coordination compound strontium nitrate with formamide and carbamide – |
|
4. Conclusions
- For the first time, various ligand coordination compounds of barium and strontium nitrates with formamide and carbamide have been synthesized. The composition, individuality, and coordination methods of ligands and nicotinic fragments have been established. The coordination nodes of complex compounds are formed with the participation of nitrate groups and have the configuration of a distorted octahedron. The research results can serve as reference data for researchers and students.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML