-
Paper Information
- Next Paper
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
International Journal of Materials and Chemistry
p-ISSN: 2166-5346 e-ISSN: 2166-5354
2025; 15(3): 59-63
doi:10.5923/j.ijmc.20251503.06
Received: Sep. 3, 2025; Accepted: Sep. 26, 2025; Published: Sep. 29, 2025

Study of Sorption of Lanthanum (III) Ions by PAN-co-DVB@DEA Anion Exchanger
Dilnoza Adinaeva1, Shakhlo Saidova1, Kholida Azizova2, Nuritdin Kattaev3, Khamdam Akbarov3
1PhD Student, Department of Physical Chemistry, National University of Uzbekistan, Tashkent, Uzbekistan
2PhD, Department of Physical Chemistry, National University of Uzbekistan, Tashkent, Uzbekistan
3DSc, Professor, Department of Physical Chemistry, National University of Uzbekistan, Tashkent, Uzbekistan
Correspondence to: Dilnoza Adinaeva, PhD Student, Department of Physical Chemistry, National University of Uzbekistan, Tashkent, Uzbekistan.
| Email: |  |
Copyright © 2025 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

The sorption of lanthanum(III) ions by a novel anion exchange resin based on polyacrylonitrile-co-divinylbenzene modified with diethanolamine (PAN-co-DVB@DEA) was systematically investigated through equilibrium, kinetic, and thermodynamic analyses. Equilibrium sorption data at 20, 30, and 40°C were fitted using Langmuir and Freundlich models. At 20 and 40°C, the Freundlich equation provided the best description (R² > 0.997), reflecting the heterogeneous nature of the sorbent surface and the predominance of physisorption. At 30°C, the Langmuir model achieved a slightly superior fit (R² = 0.9997), suggesting a tendency toward monolayer adsorption on a more uniform set of active sites. Kinetic studies at 20°C were evaluated with pseudo-first-order, pseudo-second-order, Elovich, and Weber–Morris models. The intraparticle diffusion model of Weber–Morris provided the best correlation (R² up to 0.89), indicating that diffusion of La³⁺ ions into the polymeric pores governs the sorption process, with additional contributions from boundary layer resistance. The Elovich model further confirmed surface heterogeneity and possible chemisorption contributions at the early stages of uptake. Thermodynamic parameters derived from Van’t Hoff analysis revealed negative ΔG° values (–18.4 to –21.7 kJ/mol), confirming spontaneity, while positive ΔH° (+29.5 kJ/mol) and ΔS° (+0.163 kJ/mol·K) indicated endothermic sorption accompanied by increased interfacial disorder. Overall, the results demonstrate that PAN-co-DVB@DEA is a promising sorbent for rare-earth recovery, combining high capacity, heterogeneous binding, and diffusion-driven uptake mechanisms.
Keywords: Lanthanum(III) sorption, Anion exchange resin, PAN-co-DVB, Diethanolamine modification, Adsorption isotherms, Kinetic modeling, Thermodynamic analysis
Cite this paper: Dilnoza Adinaeva, Shakhlo Saidova, Kholida Azizova, Nuritdin Kattaev, Khamdam Akbarov, Study of Sorption of Lanthanum (III) Ions by PAN-co-DVB@DEA Anion Exchanger, International Journal of Materials and Chemistry, Vol. 15 No. 3, 2025, pp. 59-63. doi: 10.5923/j.ijmc.20251503.06.
1. Introduction
- The increasing demand for rare earth elements (REEs) in advanced technologies such as electronics, renewable energy systems, catalysts, and magnetic materials has stimulated the development of efficient methods for their recovery from aqueous solutions. Lanthanum, one of the light rare earth elements, is widely used in optical materials, rechargeable batteries, and petroleum refining catalysts, making its selective separation and concentration a matter of both industrial and environmental importance. Conventional methods such as solvent extraction and precipitation often suffer from high reagent consumption, secondary pollution, and limited selectivity, thus driving the search for alternative approaches based on sorption technologies [1,2].Polyacrylonitrile (PAN) is a versatile polymer widely employed as a matrix for the preparation of sorbents due to its mechanical stability, resistance to aggressive media, and ease of chemical modification. The introduction of divinylbenzene (DVB) as a crosslinker improves the rigidity and porosity of the polymer network, creating a stable backbone suitable for functionalization [3]. Modification with diethanolamine (DEA) provides amino and hydroxyl groups capable of chelation and ion-exchange interactions with metal cations, thereby enhancing the affinity of the sorbent toward rare earth ions [4,5].To characterize the sorption properties of new sorbent materials, equilibrium, kinetic, and thermodynamic analyses are commonly applied. Classical adsorption models such as Langmuir and Freundlich allow evaluation of the homogeneity or heterogeneity of the sorption surface [6]. Kinetic models including pseudo-first-order, pseudo-second-order, Elovich, and Weber–Morris intraparticle diffusion describe the rate-controlling steps of adsorption [7]. Thermodynamic analysis based on the Van’t Hoff equation provides insight into the spontaneity, enthalpy, and entropy changes accompanying the sorption process, which are critical for understanding the mechanism and practical applicability of the sorbent [8].The objective of the present work is to study the sorption of La³⁺ ions onto a novel anion exchange resin based on PAN-co-DVB modified with diethanolamine (PAN-co-DVB@DEA). Equilibrium, kinetic, and thermodynamic approaches are combined to provide a comprehensive characterization of the sorption process and to elucidate the underlying mechanism. The findings are expected to contribute to the development of efficient polymer-based sorbents for rare earth recovery from aqueous media [9].
2. Materials and Methods
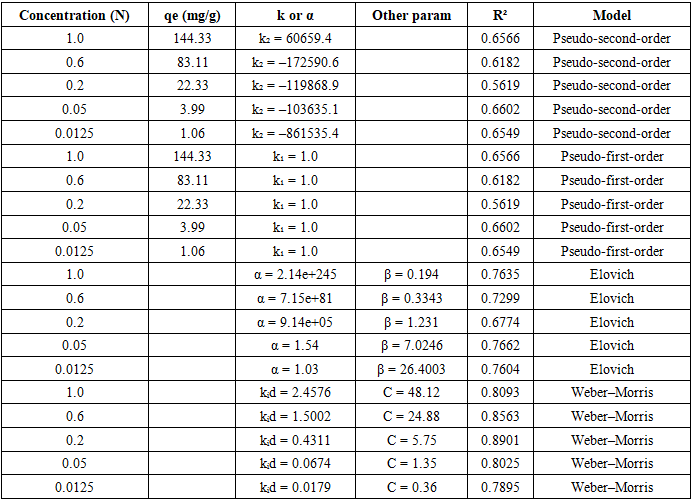
- Sorption experimentsBatch experiments were performed in a thermostated shaker (IKA KS 4000 i control, Germany) at 20, 30, and 40°C. For equilibrium isotherms, 0.1 g of resin was added to 100 mL of La³⁺ nitrate solutions (0.01–0.5 M). Contact time was 24 h to ensure equilibrium.For kinetic studies, experiments were carried out at 20°C using La³⁺ solutions of different concentrations (0.0125–1.0 N). Samples were withdrawn at specific intervals (5–180 min), filtered through 0.45 μm cellulose nitrate membranes, and analyzed.La³⁺ concentration before and after sorption was determined using atomic absorption spectroscopy (AAS, PerkinElmer AAnalyst 400) and verified by inductively coupled plasma optical emission spectroscopy (ICP-OES, Agilent 720-ES).Thermodynamic analysisExperiments were conducted at 293, 303, and 313 K with constant initial La³⁺ concentration (0.2 N). Distribution coefficients (K) were calculated, and thermodynamic parameters (ΔG°, ΔH°, ΔS°) were determined from Van’t Hoff plots.Data analysisAll isotherm, kinetic, and thermodynamic models were fitted using OriginPro 2022 (OriginLab Corp., USA) and Microsoft Excel. Regression coefficients (R²) and error functions were used to assess model adequacy.
3. Result and Discussion
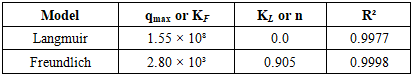
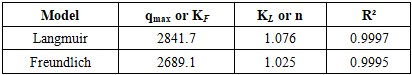
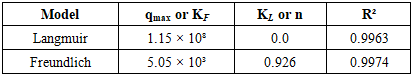
- 1. Equilibrium sorption isothermsThe equilibrium uptake of La³⁺ ions by PAN-co-DVB@DEA was investigated at 20, 30, and 40°C using the Langmuir and Freundlich adsorption models (Tables 1–3, Figs. 1). These models are frequently applied to evaluate sorption equilibria of metal ions on polymeric sorbents with heterogeneous surfaces [3,4]. The isotherms exhibited nonlinear profiles, reflecting the complex interplay between electrostatic attraction, coordination bonding with the introduced diethanolamine groups, and diffusion processes in the porous matrix.
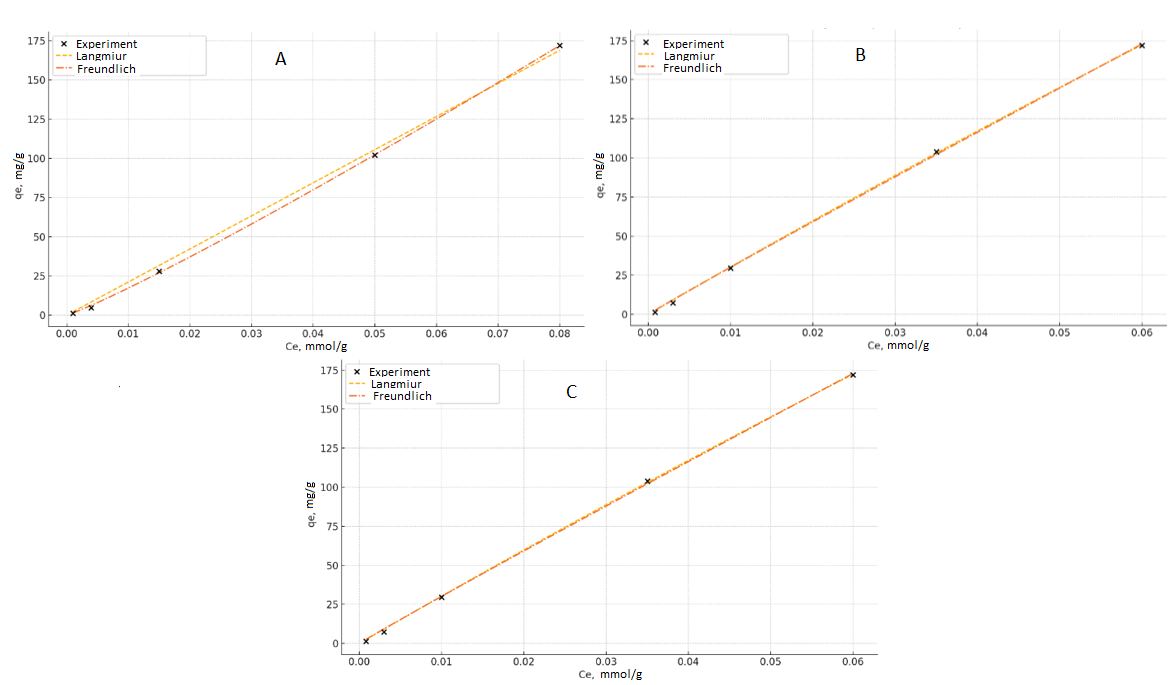 | Figure 1. Sorption isotherms of La³⁺ ions on the anion exchanger AN-DVB-DEA according to the Langmuir and Freundlich models at temperatures of 20 (A), 30 (B) and 40°C (C) |
|
|
|
|
|
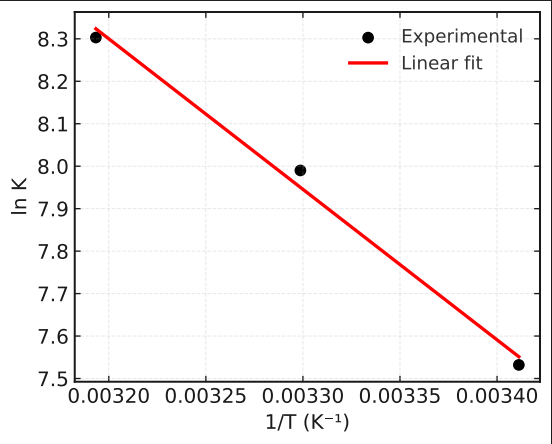 | Figure 2. Van't Hoff plot for La3+ sorption |
4. Conclusions
- The present study demonstrates that the anion exchange resin PAN-co-DVB modified with diethanolamine (PAN-co-DVB@DEA) exhibits a high affinity toward La³⁺ ions and provides valuable insights into the mechanism of sorption through equilibrium, kinetic, and thermodynamic analyses. Equilibrium studies revealed that the Freundlich model best described the isotherms at 20 and 40°C, reflecting the heterogeneous nature of the sorbent surface and the predominance of physisorption, while at 30°C the Langmuir model achieved superior correlation, suggesting a tendency toward monolayer adsorption under more uniform conditions. The temperature-dependent shift between models highlights the structural flexibility of the DEA-modified polymer and its influence on surface heterogeneity.Kinetic modeling confirmed that intraparticle diffusion is the dominant rate-limiting step, as indicated by the Weber–Morris model, while the Elovich equation further revealed contributions from heterogeneous surface binding and possible chemisorption during the early stages of uptake. Pseudo-first- and pseudo-second-order models were found to be less adequate for describing the kinetics of this system. Thermodynamic analysis showed that the sorption of La³⁺ is spontaneous (ΔG° < 0) and endothermic (ΔH° = +29.5 kJ/mol), accompanied by a positive entropy change (ΔS° = +0.163 kJ/mol·K), consistent with increased disorder at the solid–solution interface due to partial desolvation of lanthanum ions.Overall, the results indicate that PAN-co-DVB@DEA is a promising sorbent for rare earth recovery from aqueous solutions. Its performance is governed by a diffusion-limited mechanism with significant contributions from heterogeneous binding and temperature-enhanced accessibility of functional sites. These findings provide a foundation for further optimization of functionalized polymeric sorbents for selective and efficient recovery of rare earth elements.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML