Altinay Aitmuratova1, Gozzal Sidrasulieva2, Nuritdin Kattaev3, Khamdam Akbarov3
1PhD Student, Department of Physical Chemistry, National University of Uzbekistan, Tashkent, Uzbekistan
2PhD, Department of Physical Chemistry, National University of Uzbekistan, Tashkent, Uzbekistan
3DSc, Professor, Department of Physical Chemistry, National University of Uzbekistan, Tashkent, Uzbekistan
Correspondence to: Altinay Aitmuratova, PhD Student, Department of Physical Chemistry, National University of Uzbekistan, Tashkent, Uzbekistan.
| Email: |  |
Copyright © 2025 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Abstract
The adsorption properties of nanodispersed nickel oxide (NiO) obtained from spent TO-2 catalyst were studied. Low-temperature nitrogen adsorption–desorption at 77 K showed that the isotherm has an S-shaped character with a hysteresis loop of type IV according to the IUPAC classification, which indicates a mesoporous structure of the material. The BET method calculated the specific surface area to be 157.6 m²/g, while QSDFT gave a value of 120.6 m²/g with a total pore volume of 0.138 cm³/g. The main contribution to the structure was made by mesopores with a diameter of 1.5–4.0 nm (modal size ~3.3 nm), while micropores (<2 nm) made up a smaller share. The maximum sorption capacity reached 111 cm³/g at P/P₀=0.95. The isotherm approximation showed that the best agreement with the experimental data was provided by the Freundlich model (R²=0.975), which takes into account the energy heterogeneity of the surface, while the Temkin and Langmuir models demonstrated limited applicability. The results obtained confirm that nanodispersed NiO has a developed hierarchical porous structure and high sorption activity, which makes it promising for use in cryogenic sorption processes, gas separation and photocatalysis.
Keywords:
Nickel oxide (NiO), Spent catalyst recycling, Nitrogen adsorption, Mesoporous materials, Adsorption isotherms
Cite this paper: Altinay Aitmuratova, Gozzal Sidrasulieva, Nuritdin Kattaev, Khamdam Akbarov, Low-Temperature Nitrogen Adsorption on Nanodispersed NiO, International Journal of Materials and Chemistry, Vol. 15 No. 3, 2025, pp. 48-53. doi: 10.5923/j.ijmc.20251503.04.
1. Introduction
Disposing of spent catalysts from petrochemical production is an essential task of modern chemical technology, due to the need to ensure environmental safety and rational use of resources. According to data [1, pp. 45–50], tens of thousands of tons of spent nickel-containing catalysts are generated annually. The presence of nickel oxide and γ-Al₂O₃ in their composition makes them valuable secondary raw materials for producing functional materials.Nanodispersed NiO attracts the attention of researchers due to its high specific surface area, catalytic activity and potential for use in adsorption processes [2, pp. 101–107]. NiO-based materials are used in gas purification, cryosorption technologies and photocatalysis, where high dispersion and developed porous structure play a key role [3, pp. 603–619].Low-temperature nitrogen adsorption methods (77 K) are widely used to determine the textural characteristics of powders and porous bodies [4, pp. 1051–1069]. According to the IUPAC classification, type IV isotherms with hysteresis loops indicate a mesoporous structure of the material [5, pp. 145–152], which is essential for assessing its applicability in sorption and gas separation. Comparison of experimental isotherms with model dependencies (Langmuir, Freundlich, Temkin) allows us to analyse the energy heterogeneity of the surface and establish the sorption mechanism. This work aimed to study the textural characteristics and sorption behaviour of nanodispersed nickel oxide synthesised from spent catalyst TO-2 using low-temperature nitrogen adsorption methods and theoretical modelling of isotherms.
2. Materials and Methods
The low-temperature nitrogen adsorption–desorption method was used to study the textural characteristics of nanodispersed NiO obtained from the spent industrial catalyst TO-2. The research was carried out using spent catalyst of natural gas hydroreforming process TO-2, containing, depending on the batch, from 6-8% to 34-35% nickel. The spent catalyst TO-2 was dissolved by acid leaching using 30% aqueous nitric acid solution. Nickel was precipitated as nickel hydroxide by alkaline leaching with 2 M NaOH (pH 10-11) followed by calcination at 400°C for 2 hours [6, p. 295] (Figure 1).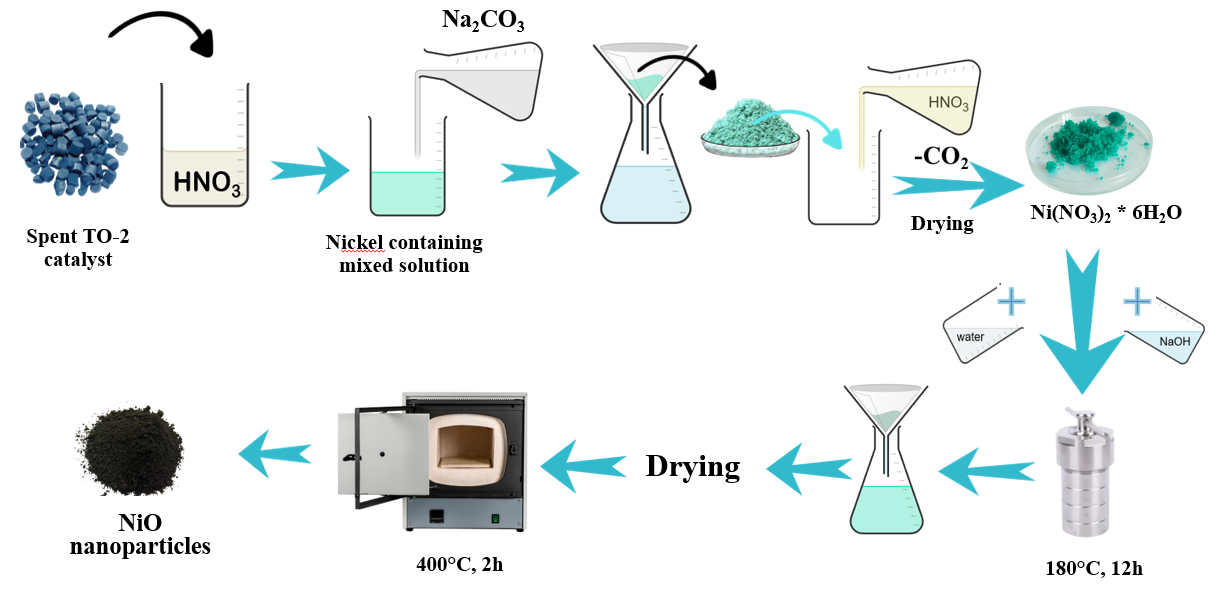 | Figure 1. Scheme for obtaining nanodispersed NiO based on spent catalyst TO-2 |
The measurements were carried out at liquid nitrogen temperature (77 K) using an automatic gas adsorption analyzer. Before the experiment, the samples were degassed at 200°C in a vacuum to remove adsorbed gases and moisture. Based on the obtained isotherms, the specific surface area was calculated using the BET method, and the pore size distribution was calculated using the BJH and QSDFT methods.Various isothermal models were used to evaluate the sorption properties. Experimental data were approximated using the Langmuir, Freundlich, and Temkin equations. The Langmuir model was used to analyze monomolecular adsorption on a homogeneous surface, the Freundlich model was used to account for surface heterogeneity, and the Temkin model was used to describe the change in adsorption energy with coating growth. The Kelvin equation was used to evaluate pore radii, which allows us to relate the relative pressure to the meniscus radius in the pore and identify the ranges of active filling. Thus, the technique included a set of successive stages: utilization of the TO-2 catalyst and isolation of NiO in a nanodispersed state, obtaining nitrogen adsorption isotherms at 77 K and processing them using modern methods of porous structure analysis. This approach allowed us to comprehensively characterize the textural parameters of the material and identify its sorption features.
3. Result and Discussion
The nitrogen adsorption–desorption isotherm recorded at 77 K on nanodispersed NiO has an S-shaped character with a pronounced hysteresis loop. According to the IUPAC classification, type IV isotherms are characteristic of mesoporous materials and describe the sequence of monomolecular and multimolecular adsorption on pore walls, followed by capillary condensation at pressures below the saturation pressure p₀ [3, pp. 603–619] [Figure 2-3].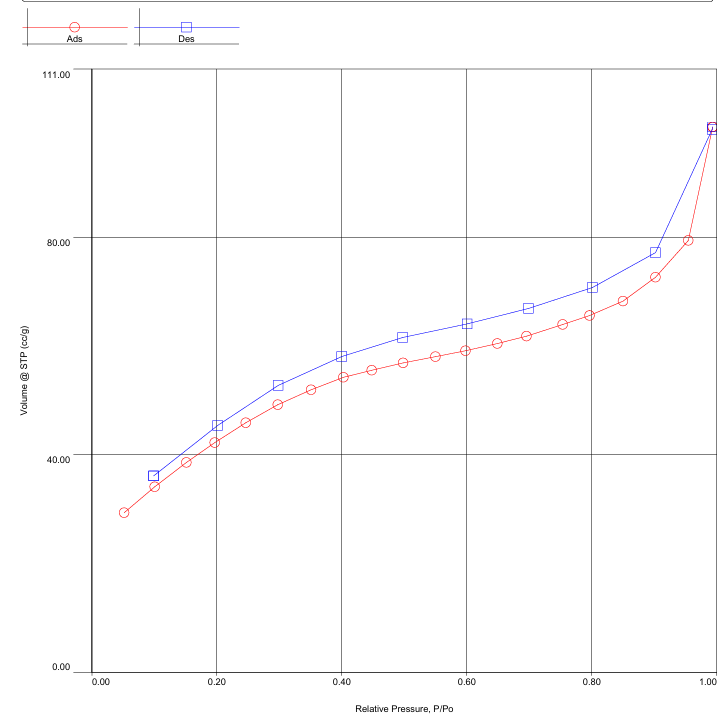 | Figure 2. Adsorption–desorption isotherm of N2 on nanodispersed NiO |
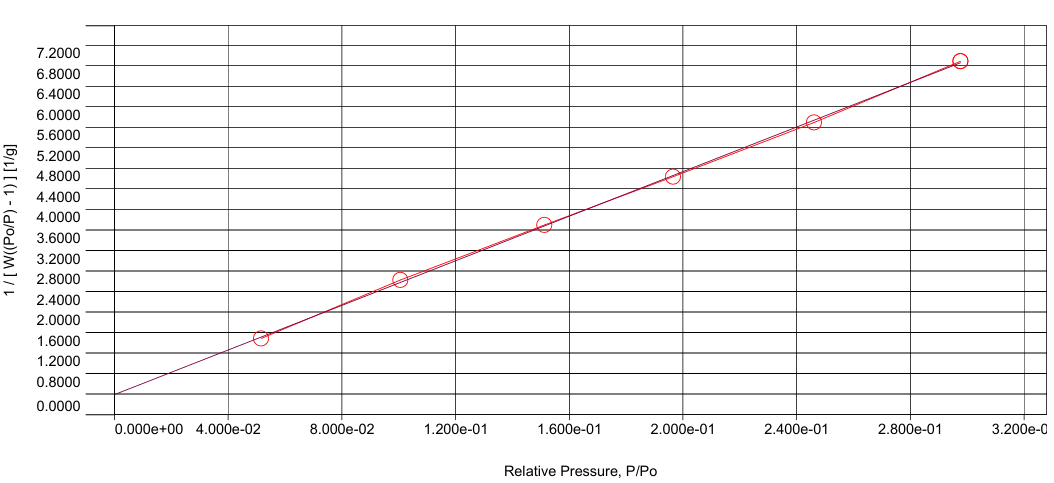 | Figure 3. Linear BET plot for N2 sorption on NiO |
Hysteresis occurs when the pore width exceeds a critical value (~4 nm for nitrogen adsorption at 77 K) and is associated with capillary condensation; for smaller pore sizes, the isotherm is completely reversible [5, pp. 145–152]. The presence of a loop in the experimental data indicates a developed mesoporous structure of NiO, and the increase in adsorption at very low pressures confirms the presence of micropores [4, pp. 1051–1069].The BET method allowed us to calculate the specific surface area equal to 157.6 m²/g, comparable with the data for nanodispersed NiO synthesized by other methods [7, pp. 312–318]. The QSDFT method, used for a more rigorous analysis, showed a specific surface area of 120.6 m²/g and a total pore volume of 0.138 cm³/g. The pore size distribution obtained for the nitrogen model in the system of slit and cylindrical pores has an intense maximum in the range of 7–20 Å, half the pore width (1.4–4.0 nm full diameter). The most significant contribution corresponds to a value of ~16.6 Å (~3.3 nm), indicating the predominance of small mesopores. With an increase in pore size to 40–50 Å, the dV(r) intensity decreases, and the presence of shoulders in the range of 80–160 Å indicates the presence of wider mesopores [8, pp. 540–547] [Figure 4].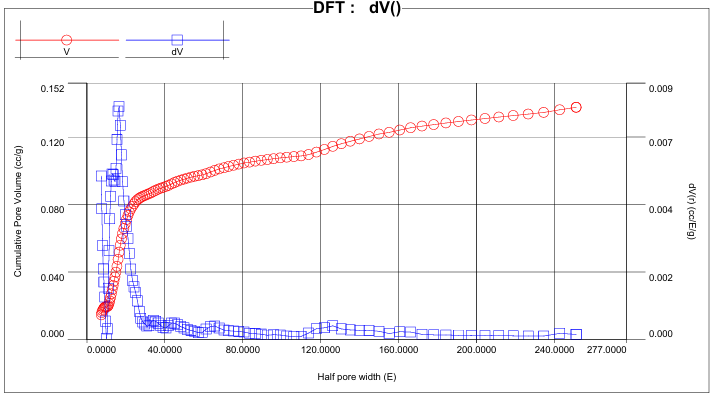 | Figure 4. Integral (V) and differential (dV) pore distributions for NiO |
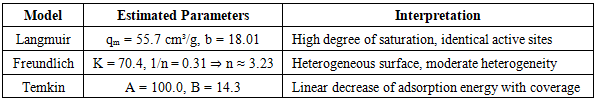
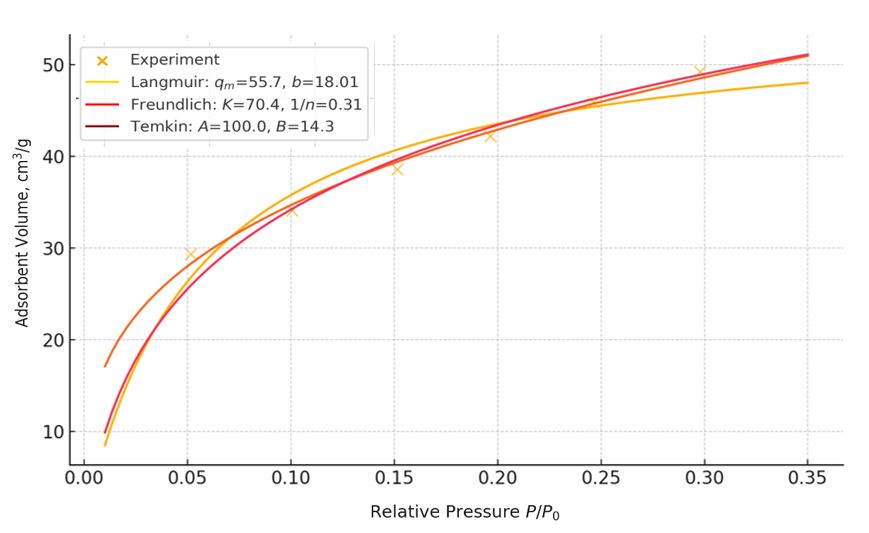
The integral distribution of pore volume showed that about 70–80% of the total volume is formed in pores with a diameter of up to 8 nm. In contrast, the contribution of micropores (<2 nm) was significantly lower. However, the micropores are responsible for the increase in adsorption at P/P₀ frankly <0.05 and contribute to the sorption of small molecules. Such a hierarchical structure (a combination of micro- and mesopores) is optimal for sorbents and catalysts, since it balances a high surface area and rapid mass transfer [9, pp. 1793–1805].The maximum adsorption volume recorded at P/P₀=0.95 was 111.0 cm³/g, confirming the material's high sorption capacity. Classical adsorption models were used to approximate the experimental data. At low pressures (P/P₀ ≤0.3), the Langmuir model yielded qₘ=55.7 cm³/g at b=18.01 and provided a satisfactory description of the process (R²=0.923, MSE=3.508). However, the overestimation of the sorption capacity in the high-pressure region indicates the limitations of this model, which is typical for systems with a wide pore structure [Tables 1-2].Table 1. Statistical parameters of adsorption isotherm modelling
 |
| |
|
Table 2. Parameters of adsorption isotherm models and their interpretation
 |
| |
|
 | Figure 5. Modelling of the isotherm of low-temperature adsorption of N2 on NiO at low relative pressures |
The Freundlich model showed the best fit to the experimental data (R²=0.989, MSE=0.486), reflecting the energetic heterogeneity of the surface; the K coefficient was 70.4, and the index 1/n=0.31 (n≈3.23) indicates a moderate heterogeneity of the sorption centers. The Temkin model, which considers the decrease in adsorption energy with increasing surface coverage, also showed a good fit (R²=0.949, MSE=2.343) and parameters A=100.0, B=14.3, characteristic of mesoporous bodies.When the analysis was extended to P/P₀=0.95, the adsorption volume increased to 111 cm³/g. In this range, the Freundlich model again provided the best agreement (R²=0.975, MSE=19.68; K=96.4, n=3.97), confirming the presence of a wide range of energy centres on the NiO surface. The Temkin model (R²=0.949, MSE=40.48) adequately described the process in the medium pressure range, and the Langmuir model (R²=0.940, MSE=46.74) deviated significantly from the experimental values at P/P₀>0.75. The Kelvin equation was used to clarify the radii of pores actively filled at different pressures, which relates the relative pressure to the meniscus radius. Calculations showed that at P/P₀=0.45–0.65, pores with a radius of 1.2–2.2 nm are actively filled, at P/P₀≈0.85, pores of 3–6 nm are involved, and at P/P₀→1, macropores >10 nm. This distribution is consistent with the QSDFT data and confirms the dominance of the narrow mesopore system (1.5–3.5 nm) with a small proportion of micropores. The specific surface area evaluation based on the model parameters showed that the Langmuir model gives an overestimated value (587.2 m²/g). In contrast, the Freundlich (214.1 m²/g) and Temkin (239.8 m²/g) models provide more realistic results than the experimental BET and QSDFT data. This indicates that the NiO surface is energetically heterogeneous and multilayer adsorption plays a significant role.Thus, a comprehensive analysis of low-temperature nitrogen adsorption, BET, QSDFT methods and model approximations showed that nanodispersed NiO obtained from the spent TO-2 catalyst has a developed hierarchical porous structure with a modal pore size of about 3.3 nm and a high specific surface area (120–160 m²/g). Sorption capacity of up to 111 cm³/g and a combination of micro- and mesopores make the material promising for cryosorption technologies, gas separation and photocatalysis.
4. Conclusions
The study showed that utilisation of the TO-2 catalyst allows obtaining nanodispersed nickel oxide with a developed porous structure. Low-temperature nitrogen adsorption at 77 K revealed a specific surface area of 120–160 m²/g, a total pore volume of 0.138 cm³/g, and a modal size of about 3.3 nm, indicating the predominance of mesopores with a partial contribution of micropores. The maximum sorption capacity reached 111 cm³/g at P/P₀=0.95.A comparative analysis of the models showed that the best description of the isotherm is given by the Freundlich model (R²=0.975), reflecting the energy heterogeneity of the surface. In contrast, the Langmuir and Temkin models have limited applicability. Thus, nanodispersed NiO obtained from spent catalyst TO-2 combines a high specific surface area, hierarchical porosity and significant sorption capacity, which makes it promising for cryogenic sorption processes, gas separation and photocatalysis.
References
| [1] | Gates, B. C. (1992). Catalytic Chemistry. Wiley, New York, 45–50. |
| [2] | Murphy, A. B. (2014). Semiconducting Metal Oxides as Gas-Sensing Materials, 101–107. |
| [3] | Sing, K. S. W., Everett, D. H., Haul, R. A. W., Moscou, L., Pierotti, R. A., Rouquérol, J., & Siemieniewska, T. (1985). Reporting physisorption data for gas/solid systems with special reference to the determination of surface area and porosity. Pure and Applied Chemistry, 57(4), 603–619. https://doi.org/10.1351/pac198557040603. |
| [4] | Thommes, M., Kaneko, K., Neimark, A. V., Olivier, J. P., Rodriguez-Reinoso, F., Rouquerol, J., & Sing, K. S. W. (2015). Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC Technical Report). Pure and Applied Chemistry, 87(9–10), 1051–1069. https://doi.org/10.1515/pac-2014-1117. |
| [5] | Rouquerol, J., Rouquerol, F., & Sing, K. (1999). Adsorption by Powders and Porous Solids. Academic Press, London, 145–152. |
| [6] | Aitmuratova, A., Sidrasulieva, G., Kattaev, N., & Akbarov, Kh. (2025). Preparation and study of structural–morphological properties of nanosized NiO. Vestnik NUUz, 3(1). 290-293. |
| [7] | Luo, J., Meng, M., & Li, X. (2011). Synthesis of NiO nanoparticles and their adsorption performance. Microporous and Mesoporous Materials, 145(1–3), 312–318. https://doi.org/10.1016/j.micromeso.2011.05.025. |
| [8] | Chen, L., Jiang, H., & Li, C. (2012). Mesoporous nickel oxide with tunable porosity for adsorption applications. Journal of Colloid and Interface Science, 380(2), 540–547. https://doi.org/10.1016/j.jcis.2012.05.019. |
| [9] | Lu, A. H., & Schüth, F. (2006). Nanocasting: A versatile strategy for creating nanostructured porous materials. Advanced Materials, 18(14), 1793–1805. https://doi.org/10.1002/adma.200600148. |







 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML
