-
Paper Information
- Next Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
International Journal of Materials and Chemistry
p-ISSN: 2166-5346 e-ISSN: 2166-5354
2025; 15(3): 41-47
doi:10.5923/j.ijmc.20251503.03
Received: Aug. 21, 2025; Accepted: Sep. 19, 2025; Published: Sep. 29, 2025

Adsorption Performance and Thermodynamic Analysis of Silica–Bentonite Composite for Methylene Blue Removal
SarvarbekKoraev1, Rayhona Muassarova2, Gozzal Sidrasulieva3, Nuritdin Kattaev4, Khamdam Akbarov4
1PhD Student, Department of General Chemistry and Chemical Technologies, Denau Institute of Entrepreneurship and Pedagogy, 190507 Denau Sharof Rashidov 360, Republic of Uzbekistan
2PhD Student, Department of Physical Chemistry, National University of Uzbekistan, Tashkent, Uzbekistan
3PhD, Department of Physical Chemistry, National University of Uzbekistan, Tashkent, Uzbekistan
4DSc, Professor, Department of Physical Chemistry, National University of Uzbekistan, Tashkent, Uzbekistan
Correspondence to: SarvarbekKoraev, PhD Student, Department of General Chemistry and Chemical Technologies, Denau Institute of Entrepreneurship and Pedagogy, 190507 Denau Sharof Rashidov 360, Republic of Uzbekistan.
| Email: |  |
Copyright © 2025 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

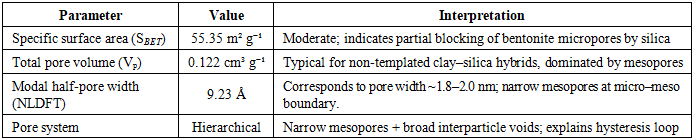
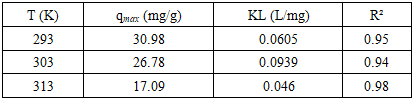
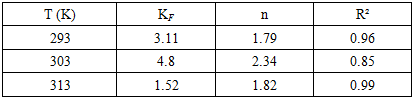
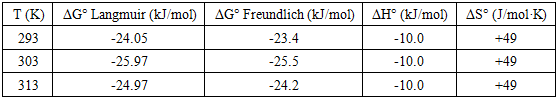
The increasing discharge of synthetic dyes into water bodies poses severe environmental risks due to their toxicity, persistence, and resistance to biodegradation. Methylene blue (MB), a widely used cationic dye, is often chosen as a model pollutant for adsorption studies. This work synthesised a bentonite–silica composite via a sol–gel method using tetraethoxysilane (TEOS) as a silica precursor and natural Navbahor bentonite as the clay component. The composite was characterized by Fourier-transform infrared spectroscopy (FTIR) and nitrogen adsorption–desorption analysis (BET, NLDFT). FTIR confirmed the incorporation of an amorphous silica network into the bentonite matrix, while maintaining the layered montmorillonite structure. Textural analysis revealed a surface area of 55.35 m²/g⁻¹, a pore volume of 0.122 cm³/g⁻¹, and a dominant pore size of ~1.8–2.0 nm, indicating a hierarchical mesoporous system favourable for adsorption and diffusion. The adsorption of MB was investigated at different temperatures. Langmuir fitting yielded maximum capacities of 30.98, 26.78, and 17.09 mg g⁻¹ at 293, 303, and 313 K, respectively, confirming an exothermic process. Freundlich analysis emphasised surface heterogeneity, with n > 1 and correlation coefficients up to 0.99. Thermodynamic calculations indicated negative Gibbs free energies (−24 to −26 kJ mol⁻¹), an enthalpy change of −10 kJ mol⁻¹, and positive entropy values (+45–50 J mol⁻¹ K⁻¹). These findings demonstrate that MB adsorption is spontaneous, exothermic, and governed by a dual mechanism combining monolayer adsorption on silica-modified sites and heterogeneous sorption in bentonite domains.
Keywords: Bentonite–silica composite, Methylene blue adsorption, Sol–gel synthesis, Isotherm models, Thermodynamic analysis
Cite this paper: SarvarbekKoraev, Rayhona Muassarova, Gozzal Sidrasulieva, Nuritdin Kattaev, Khamdam Akbarov, Adsorption Performance and Thermodynamic Analysis of Silica–Bentonite Composite for Methylene Blue Removal, International Journal of Materials and Chemistry, Vol. 15 No. 3, 2025, pp. 41-47. doi: 10.5923/j.ijmc.20251503.03.
1. Introduction
- Water pollution caused by synthetic dyes is a serious environmental concern due to their extensive use in textile, leather, paper, and other industries. Methylene blue (MB) is often employed as a model organic dye because of its high stability, toxicity, and resistance to biodegradation. Even at low concentrations, MB can reduce light penetration in aquatic environments, inhibit photosynthesis, and pose health risks to living organisms [1–3]. Therefore, developing efficient and cost-effective adsorbents for MB removal from wastewater remains an urgent scientific and technological task.Natural clay minerals, particularly bentonite, are among the most widely used sorbents owing to their low cost, wide availability, high cation-exchange capacity, and layered structure that enables adsorption of various organic and inorganic pollutants [4,5]. However, pristine bentonite often has limited surface area and pore accessibility, restricting its adsorption capacity toward larger organic molecules such as dyes. To overcome these limitations, bentonite is frequently modified with other materials to create hybrid composites with enhanced textural and surface properties [6,7].Silica (SiO₂) is an attractive modifier because it provides high surface area, chemical stability, and abundant silanol groups capable of interacting with pollutants. Incorporating silica into bentonite matrices can stabilize the clay structure, prevent layer collapse, and create additional porosity, thus improving adsorption performance [8]. Among various silica precursors, tetraethoxysilane (TEOS) is widely used in sol–gel synthesis because it can undergo controlled hydrolysis and condensation, forming homogeneous silica networks [9].Recent studies have demonstrated that bentonite–silica composites exhibit improved adsorption capacities for heavy metal ions and dyes compared to raw bentonite. For instance, Zhou et al. [10] showed enhanced uptake of organic pollutants on clay–silica hybrids, while Zhao et al. [11] reported significant improvement in dye removal efficiency after silica modification of bentonite. Li et al. [12] developed bentonite–silica composites via sol–gel processing and observed that the introduction of silica increased mesoporosity and sorption efficiency toward methylene blue. These findings confirm that bentonite–silica hybrids can serve as promising adsorbents for wastewater treatment.Nevertheless, adsorption mechanisms, isotherm behaviour, and thermodynamic properties strongly depend on the synthesis method, precursor ratio, and properties of the clay source. In particular, the use of local bentonite deposits combined with controlled sol–gel modification offers an opportunity to create low-cost, efficient adsorbents tailored for specific environmental applications.In this work, a bentonite–silica composite was synthesised via a sol–gel method using natural Navbahor bentonite and TEOS as the silica precursor. The composite was comprehensively characterized by Fourier-transform infrared spectroscopy (FTIR) and low-temperature nitrogen adsorption–desorption analysis (BET, NLDFT). The adsorption performance was evaluated using methylene blue as a model dye pollutant, and the equilibrium data were analysed using Langmuir and the Freundlich isotherm models. Thermodynamic parameters (ΔG°, ΔH°, ΔS°) were also calculated to gain deeper insight into the adsorption mechanism.This study aims to elucidate the relationship between the structural properties of the TEOS–bentonite composite and its adsorption behaviour toward methylene blue, thereby demonstrating its potential as a low-cost, efficient adsorbent for wastewater treatment.
2. Materials and Methods
- Materials. Tetraethoxysilane (TEOS, Si(OC₂H₅)₄, analytical grade) was used as a silica precursor without further purification. Ethanol and distilled water were applied as solvents. Natural Navbahor bentonite, preliminarily ground and sieved to <100 μm, served as the aluminosilicate component. All other reagents were of analytical grade.Synthesis of bentonite–silica composite. The bentonite–silica composite was synthesised via a sol–gel process using TEOS as the silica source. A water–ethanol mixture was adjusted to pH ≈ 10–11 with aqueous ammonia under vigorous stirring. The calculated amount of TEOS was introduced dropwise, and the reaction mixture was heated to 60°C. After 15–20 min, when the formation of a white gel-like precipitate was observed, Navbahor bentonite was gradually added in portions at a mass ratio of TEOS/bentonite = 10:1. The reaction was continued for an additional 30–40 min at 60°C under vigorous stirring to ensure homogeneous incorporation of the silica precursor into the clay matrix (Figure 1).
 | Figure 1. Scheme for Obtaining a Silica-Bentonite Composite |
3. Results and Discussion
- FTIR Analysis of the Bentonite–Silica CompositeThe FTIR spectrum of the bentonite–silica composite obtained using tetraethoxysilane (TEOS) as a precursor (Figure X) shows the combined features of montmorillonite and amorphous silica. A broad absorption band at 3700–3400 cm⁻¹ corresponds to the stretching vibrations of hydroxyl groups, including structural –OH groups of bentonite and silanol groups (Si–OH) of the silica phase. The band near 1630 cm⁻¹ is attributed to the bending vibrations of molecular water located in the interlayer space of bentonite (Figure 2).
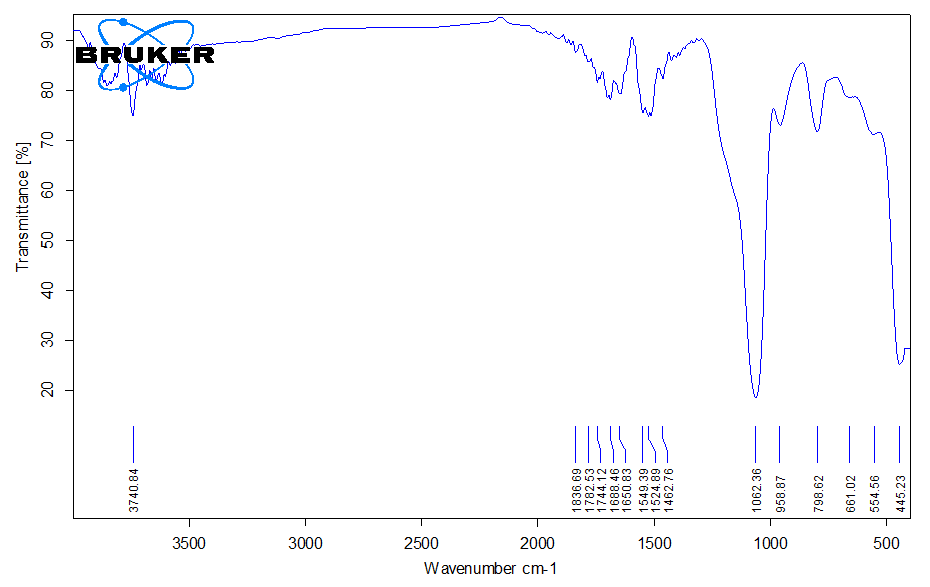 | Figure 2. FTIR spectrum of the bentonite–silica composite |
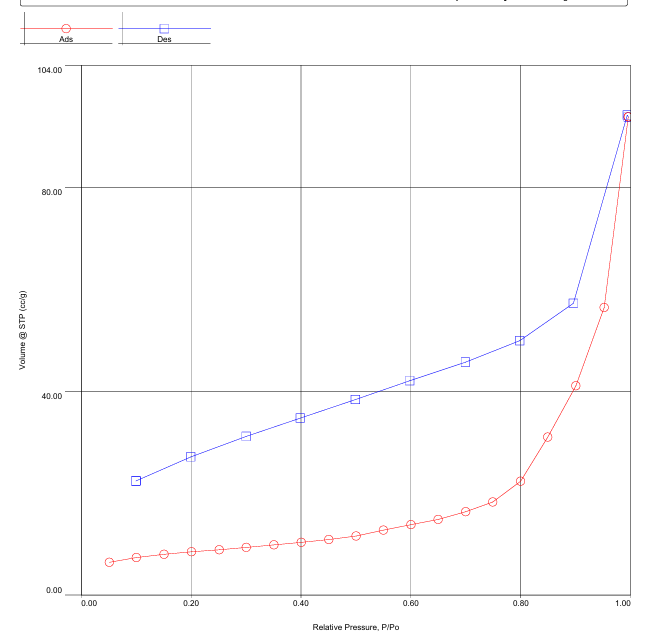 | Figure 3. Low-temperature adsorption-desorption of N2 on bentonite-silica composite |
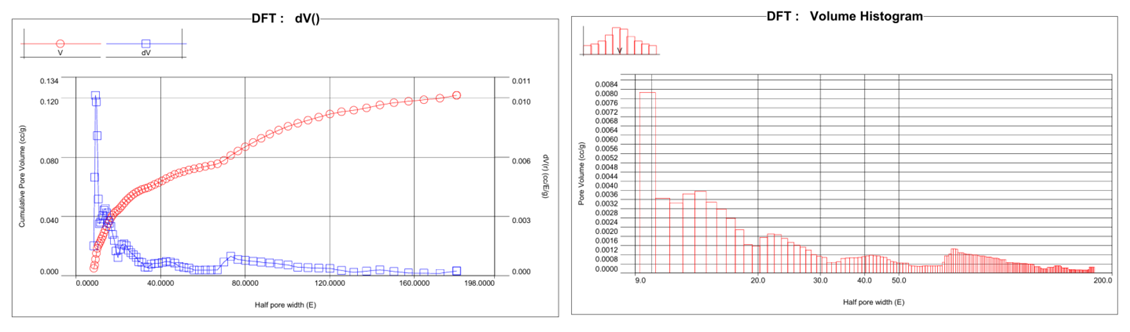 | Figure 4. Cumulative pore volume (A) and pore volume (B) distribution |
|
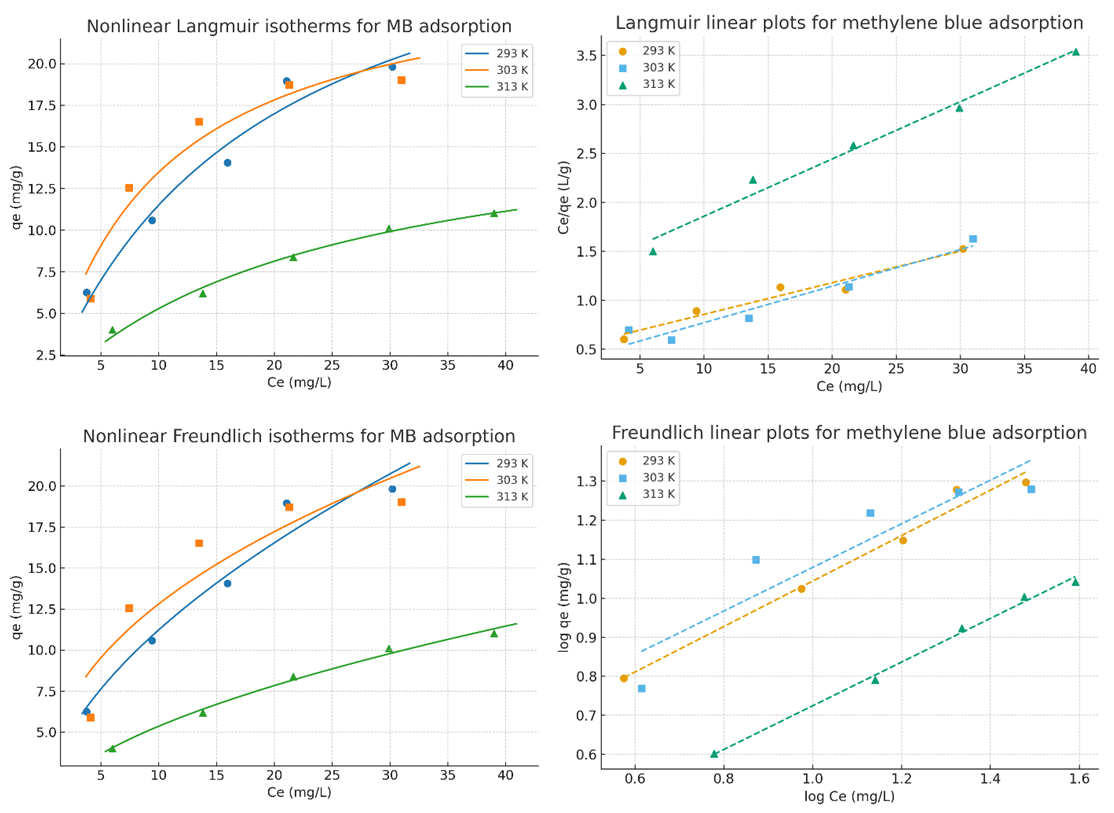 | Figure 5. Adsorption isotherms of MB on TEOS-bentonite composite and Langmuir/Freundlich linear plots for methylene blue adsorption |
|
|
|
4. Conclusions
- A bentonite–silica composite was successfully synthesised via a sol–gel process using tetraethoxysilane (TEOS) and natural Navbahor bentonite. Characterization confirmed the formation of an amorphous silica network within the clay matrix while preserving the structural features of montmorillonite. FTIR analysis revealed the coexistence of Si–O–Si vibrations typical of silica and Al–O–Si bands characteristic of bentonite. Nitrogen adsorption–desorption analysis demonstrated that TEOS modification transformed the material from predominantly microporous to mesoporous, with a surface area of 55.35 m²·g⁻¹, a pore volume of 0.122 cm³·g⁻¹, and a hierarchical pore system that improves adsorption and diffusion.Adsorption studies using methylene blue (MB) as a model dye pollutant showed maximum adsorption capacities of 30.98, 26.78, and 17.09 mg g⁻¹ at 293, 303, and 313 K, respectively. Langmuir fitting indicated monolayer adsorption with high correlation coefficients, particularly at 303 K, while the Freundlich analysis confirmed surface heterogeneity with n > 1, indicating favourable sorption. Thermodynamic parameters (ΔG° = −24 to −26 kJ mol⁻¹, ΔH° ≈ −10 kJ mol⁻¹, ΔS° ≈ +45–50 J mol⁻¹ K⁻¹) verified that adsorption is spontaneous, exothermic, and dominated by physisorption.Overall, MB uptake on the TEOS–bentonite composite proceeds via a dual mechanism: monolayer adsorption on silica-modified sites and heterogeneous sorption in bentonite domains. This synergy enhances adsorption efficiency and stability, demonstrating that TEOS–bentonite composites are promising, low-cost, and eco-friendly adsorbents for wastewater treatment. Such materials, derived from abundant natural resources and prepared via a simple sol–gel method, can contribute significantly to sustainable environmental remediation technologies.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML