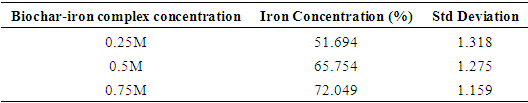-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
International Journal of Materials and Chemistry
p-ISSN: 2166-5346 e-ISSN: 2166-5354
2025; 15(3): 35-40
doi:10.5923/j.ijmc.20251503.02
Received: Jul. 17, 2025; Accepted: Aug. 3, 2025; Published: Aug. 8, 2025

Synthesis and Characterization of Biochar–Iron Complex Derived from Sugarcane Bagasse for Iron Nutrient Retention
Meshack Mutungi Kamaau1, Harun Mbuvi1, Francis Maingi2
1Department of Chemistry, Kenyatta University, Nairobi, Kenya
2Department of Science Technology and Engineering, Kibabii University, Bungoma, Kenya
Correspondence to: Francis Maingi, Department of Science Technology and Engineering, Kibabii University, Bungoma, Kenya.
| Email: |  |
Copyright © 2025 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Iron deficiency in alkaline soils poses a significant challenge to crop productivity due to limited iron availability and poor nutrient retention. Conventional fertilizers often fail to address this effectively and sustainably. This research was geared towards utilizing biochar as a chelating agent in the synthesis of biochar-iron complexes. Biochar–iron complexes were synthesized via pyrolysis, where biochar derived from sugarcane bagasse was treated with FeCl₃ at different concentrations (0.25 M, 0.5 M and 0.75 M). FT-IR analysis confirmed the successful incorporation of iron into the biochar matrix across all FeCl3 concentrations used. There were noticeable peak shifts in hydroxyl (–OH) and carboxyl (–COOH) functional groups, indicating strong chelation between iron ions and the biochar surface. The appearance of new bands at 671.23 cm⁻¹ and 507.28 cm⁻¹ emanating from Fe–O vibrations upon complexation is a clear indication of successful incorporation of iron into the biochar structure. XRF analysis quantitatively demonstrated enhanced iron retention. The study will therefore form a base for applying the biochar-iron complex synthesized as an alternative iron-rich fertilizer to enhance iron uptake in the spinach crop.
Keywords: Biochar, Characterization, Complexation, Nutrient Retention, Synthesis
Cite this paper: Meshack Mutungi Kamaau, Harun Mbuvi, Francis Maingi, Synthesis and Characterization of Biochar–Iron Complex Derived from Sugarcane Bagasse for Iron Nutrient Retention, International Journal of Materials and Chemistry, Vol. 15 No. 3, 2025, pp. 35-40. doi: 10.5923/j.ijmc.20251503.02.
Article Outline
1. Introduction
- Iron deficiency is a critical challenge in many agricultural systems, particularly in alkaline soils where iron becomes insoluble and unavailable to plants [1] [2]. Conventional iron fertilizers often suffer from rapid precipitation and immobilization, limiting their effectiveness. Biochar, produced via the pyrolysis of organic waste materials, has emerged as a promising soil amendment due to its high porosity and abundance of functional groups that facilitate nutrient retention and slow release [3] [4]. Sugarcane bagasse, an abundant agricultural by-product, is an ideal feedstock for biochar production. Converting bagasse into biochar not only reduces waste but also creates a value-added product that can enhance soil fertility. Despite these advantages, limited studies have explored the synthesis of biochar–iron complexes using sugarcane bagasse and their potential for improved nutrient retention. A review of existing studies shows that the majority of research has focused on the use of Fe-modified bagasse biochar for pollutant remediation such as phosphates, arsenic, antibiotics, pesticides [5] [6] rather than direct evaluation of its role as an iron nutrient source. The optimization of preparation methods remains another research frontier. The iron loading concentration can dramatically influence surface chemistry, pore structure, and the oxidation state of iron, all of which determine nutrient release dynamics [7]. This study addresses this gap by synthesizing and characterizing biochar–iron complexes with varying FeCl3 concentrations. Iron deficiency is a prevalent issue in alkaline soils, leading to reduced crop yields, poor plant health, and diminished nutritional quality. Traditional iron fertilizers are often ineffective in such soils due to their rapid precipitation and immobilization, resulting in poor nutrient use efficiency and high environmental losses. Although biochar has emerged as a promising soil amendment, most studies have focused on its general nutrient retention and carbon sequestration benefits. A number of studies have demonstrated the potential of biochar to enhance nutrient retention through its porous structure and functional groups [8] [9] [10]. The biochar produced from biomass such as sugarcane bagasse is rich in hydroxyl (–OH), carboxyl (–COOH), and phenolic groups—functional moieties that play key roles in metal adsorption and chelation [3]. Research by [11] showed that these oxygenated groups facilitate the formation of stable complexes with iron, effectively reducing leaching in alkaline soils. Further, [4] reported that the efficiency of such complexation is significantly influenced by the concentration of the iron precursor. In synthesis protocols, [12] optimized conditions for mixing and reacting biochar with FeCl₃, laying a strong foundation for subsequent studies aiming at enhanced iron loading. Together, these studies underscore the potential to improve iron availability in soil through strategic biochar modification, a concept that this work further investigates through detailed characterization. This study utilized biochar from sugarcane bagasse as a chelating agent to enhance iron availability and uptake in Spinacia oleracea. By investigating the complexation of iron with sugarcane bagasse–derived biochar, this study aimed to offer insights into an innovative, sustainable approach for improving iron nutrition in spinach crops grown in iron-deficient soils.
2. Materials and Methods
2.1. Biochar Preparation
- Sugarcane bagasse was collected from Mtito Andei Market, washed thoroughly with distilled water, and oven-dried at 105°C for 24 hours [13]. The dried bagasse was pyrolyzed in a Thermo Scientific muffle furnace under conditions optimized from the literature [14] to produce biochar with high carbon content and well-preserved functional groups.
2.2. Preparation of Biochar–Iron Complexes
- The produced biochar was sieved to ensure uniform particle size and divided into three portions. Each portion was mixed with an aqueous solution of FeCl₃ at concentrations of 0.25 M, 0.50 M, and 0.75 M, using weight-to-volume ratios of 1:5, 1:10, and 1:15, respectively. The mixtures were agitated at room temperature for 24 hours, filtered, washed with deionized water, and then dried at 105°C for 12 hours [12].
2.3. Characterization Techniques
2.3.1. Functional Group Analysis Using Fourier Transform Infrared Spectroscopy
- FT-IR was performed on the raw biochar and each biochar–iron complex to identify functional groups. Samples were prepared as KBr pellets, and spectra were recorded over the range 4000–400 cm⁻¹. Shifts in characteristic peaks (e.g., –OH and –COOH) were used to confirm iron chelation [15].
2.3.2. X-Ray Fluorescence of Biochar–Iron Complexes
- XRF analysis quantified the iron content retained in the biochar–iron complexes. Powdered samples were analyzed using an XRF spectrometer, and the iron content was reported as a percentage by weight with calculated standard deviations [16].
3. Results and Discussions
3.1. Characterization of Sugarcane Bagasse-Derived Biochar Using FT-IR
- The FT-IR spectra provided key understanding into the chemical interactions between biochar and iron at varying concentrations. The absorption peaks in FT-IR analysis confirmed the presence of functional groups such as hydroxyl (–OH) and carboxyl (–COOH), which are vital for iron binding [11]. These results align with previous findings indicating that biochar’s high surface functionality facilitates metal adsorption, improving its suitability for soil amendment [17]. The FT-IR spectra of the sugarcane bagasse-derived biochar revealed strong absorption bands corresponding to hydroxyl (–OH), carboxyl (–COOH), and phenolic (–PhOH) groups. These functional groups are essential for iron binding during complexation and play a role in nutrient retention, water adsorption, and buffering soil pH. Their presence demonstrates that pyrolysis preserved important surface functionalities critical for biochar’s performance as a soil amendment. In terms of synthesis, this study aligns with the general methodologies reported in literature of pyrolysis of sugarcane bagasse followed by iron impregnation using FeCl₃ [18]. However, this study uniquely varies FeCl₃ concentration (0.25 M, 0.50 M, 0.75 M) to directly assess iron loading efficiency. Most other studies employ a single concentration or mass ratio optimized for adsorption performance, without systematically examining how metal salt concentration influences nutrient release profiles for agricultural uptake [18].The FT-IR spectrum of sugarcane bagasse-derived biochar, highlights its functional groups that are critical for nutrient retention and metal chelation. The broad absorption at 3440.10 cm⁻¹ corresponds to hydroxyl (–OH) stretching vibrations, primarily from residual cellulose, hemicellulose, and lignin. These hydroxyl groups enhance biochar’s hydrophilicity and ability to form hydrogen bonds, improving water retention and facilitating metal adsorption. The peak at 1383.95 cm⁻¹ is attributed to carboxyl (–COOH) groups, which provide negatively charged sites for binding metal ions like Fe³⁺ through electrostatic attraction or chelation. Similarly, the band near 1117.77 cm⁻¹ corresponds to phenolic –OH or ether functionalities, further contributing to cation exchange capacity. The presence of these oxygen-containing groups confirms that pyrolysis preserved essential surface functionalities, which are vital for subsequent iron complexation. These observations agree with previous studies [11] [19] that report similar retention of functional groups in lignocellulosic biochars. Overall, Figure 1 validates the chemical suitability of the synthesized biochar for iron loading and soil fertility applications.
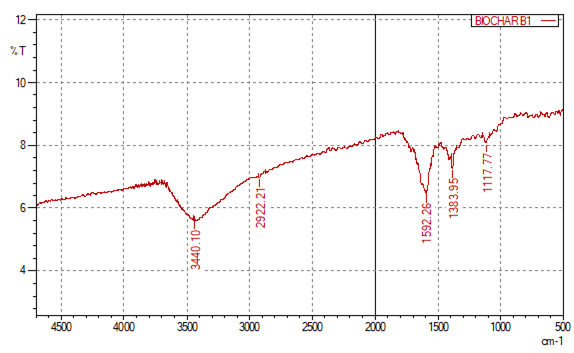 | Figure 1. Characterization of Sugarcane Bagasse-Derived Biochar Using FT-IR |
3.2. Characterization of the Biochar-Iron Complex Using FT-IR
- FT-IR analysis confirmed successful iron incorporation into the biochar matrix across all FeCl₃ concentrations. Noticeable peak shifts in hydroxyl (–OH) and carboxyl (–COOH) functional groups indicated strong chelation between iron ions and the biochar surface. The formation of Fe–O bonds was also evident in the spectra, confirming direct chemical bonding and improved stability. These changes reflect the modification of biochar’s surface chemistry without compromising its structural integrity, consistent with previous reports [4] [12].
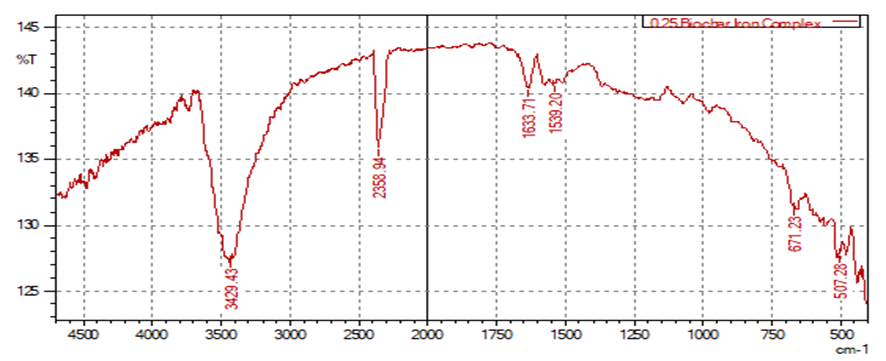 | Figure 2. Characterization of Biochar-Iron Complex Using FT-IR (0.25 M Fecl3 (1:5w/V)) |
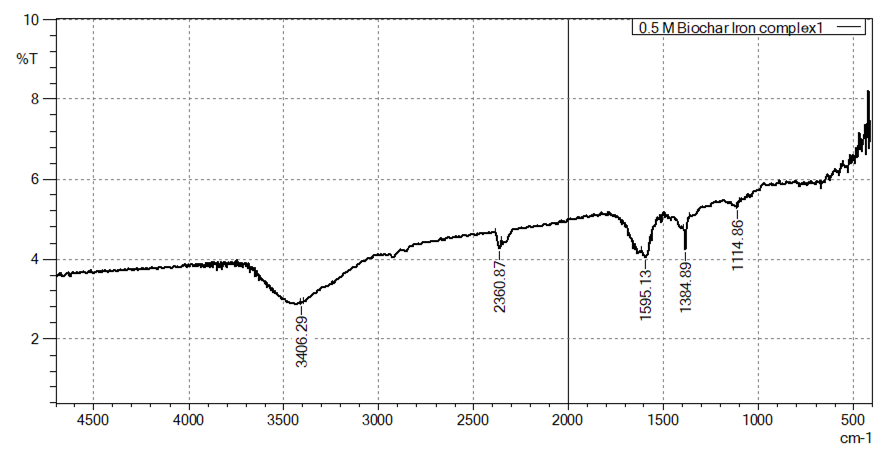 | Figure 3. Characterization of Biochar-Iron Complex Using FT-IR (0.5 M Fecl3 (1:10w/V)) |
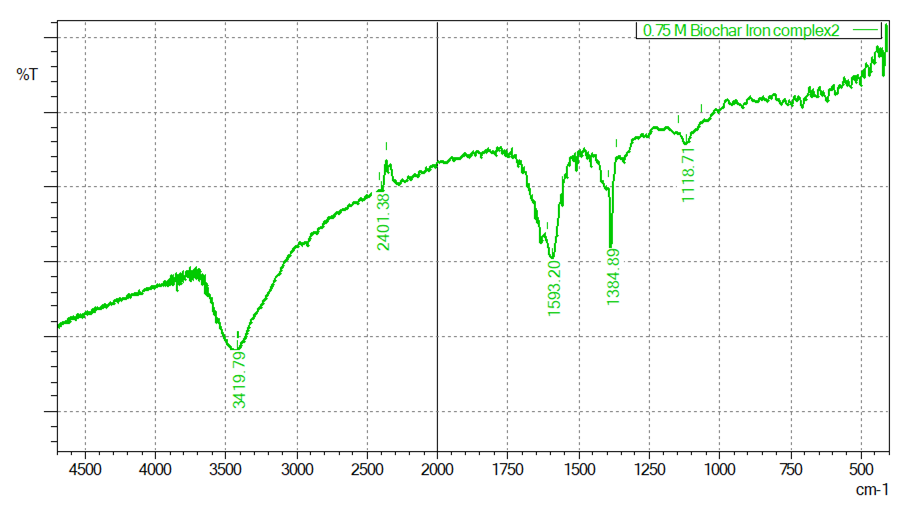 | Figure 4. Characterization of Biochar-Iron Complex Using FT-IR (0.75 M Fecl3 (1:15w/V) |
3.3. XRF Analysis of Iron Levels in the Biochar-Iron Complex
- XRF analysis quantified the incorporation of iron into the biochar matrix, showing a clear trend of increased iron retention with higher FeCl₃ concentrations. Specifically, iron content rose from 51.694% at 0.25 M to 65.754% at 0.5 M and reached 72.049% at 0.75 M (Table 1). Standard deviation values decreased slightly (from 1.318 to 1.159) as concentration increased, indicating more uniform iron deposition at higher molarities. This suggests that increasing FeCl₃ concentration enhances iron loading through improved ion-exchange interactions and surface complexation. However, the incremental gain between 0.5 M and 0.75 M (from 65.754% to 72.049%) was less pronounced than between 0.25 M and 0.5 M, indicating a potential saturation point or limited availability of reactive sites. These findings align with studies by [25], where excessive precursor concentrations yield diminishing returns due to steric hindrance or precipitation on the surface, and [26], which observed higher iron sorption capacities in biochar composites treated with elevated FeCl₃ levels. Therefore, while 0.75 M achieves maximum loading, 0.5 M may represent an optimal compromise between iron enrichment and material efficiency. These findings underscore the importance of dosage optimization for practical soil amendment use, balancing cost, performance, and environmental considerations.
|
4. Conclusions
- This study successfully synthesized sugarcane bagasse–derived biochar and complexed it with FeCl₃ to form a biochar–iron complex. FT-IR analysis confirmed the binding of iron ions to key functional groups on the biochar, and XRF analysis quantitatively demonstrated enhanced iron retention, particularly at the 0.75 M concentration. The biochar–iron complex shows significant potential as a sustainable soil amendment for improving nutrient retention and could contribute to enhanced soil fertility and crop productivity. Overall, these results reinforce the growing body of evidence that Fe-modified biochars are multifunctional materials capable of nutrient retention. However, whereas prior research has concentrated on macronutrient retention (particularly phosphorus) or contaminant immobilization, this study directly targets micronutrient enhancement in crop production. This represents a critical step toward addressing iron deficiency in leafy vegetables and developing biochar-based fertilizers tailored to micronutrient delivery.
ACKNOWLEDGEMENTS
- The authors express gratitude to Kenyatta University's department of chemistry and department of science technology and engineering of Kibabii University for the assistance offered during the research period.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML