-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
International Journal of Materials and Chemistry
p-ISSN: 2166-5346 e-ISSN: 2166-5354
2025; 15(3): 25-34
doi:10.5923/j.ijmc.20251503.01
Received: Jul. 8, 2025; Accepted: Jul. 26, 2025; Published: Jul. 30, 2025

The Effect of Various Sodium Compounds and Their Concentrations on the Synthesis of High-Molecular Hydrocarbons from CO and H₂
Asliddin Mamatov1, Hayitali Ibodullayev2, Normurot Fayzullaev3
1Department of Inorganic Chemistry and Materials Science, Samarkand State University named after Sharof Rashidov, Samarkand, Uzbekistan
2Faculty of Chemistry, Samarkand State University named after Sharof Rashidov, Institute of Biochemistry, Samarkand, Uzbekistan
3Department of Polymer Chemistry and Chemical Technology, Samarkand State University named after Sharof Rashidov, Samarkand, Uzbekistan
Correspondence to: Asliddin Mamatov, Department of Inorganic Chemistry and Materials Science, Samarkand State University named after Sharof Rashidov, Samarkand, Uzbekistan.
| Email: |  |
Copyright © 2025 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

This study investigates the effect of different sodium compounds and their concentrations on the Fischer–Tropsch synthesis (FTS) of high-molecular-weight hydrocarbons from syngas (CO + H₂) using 20%Co–20%Fe–5%B–1.5%Zr(x%Na)/Al₂O₃ catalysts. Experiments were carried out at 200–220°C, 10 atm pressure, and a space velocity of 100 h⁻¹. Catalysts were prepared by impregnation of nitrate salts onto γ-Al₂O₃, followed by calcination and reduction in hydrogen at 400°C. The products were analysed by gas chromatography. The results showed that NaNO₃-containing catalysts achieved the highest CO conversion (82–83%) and liquid C₅⁺ yield (138–147 g/m³). Na₂CO₃ increased C₅⁺ selectivity up to 91% and suppressed methanation. NaOH enhanced long-chain alkane formation with an α-value of 0.89. In contrast, NaCl reduced both activity and selectivity. An optimal sodium concentration of 1 mol% maximised C₅⁺ selectivity (92%) and minimised gas by-products, while higher concentrations (2–5%) reduced performance. Among alkali metals (Li, Na, K, Rb, Cs), sodium provided the best overall catalytic behaviour. Cs and Na also yielded the highest α-values. These findings demonstrate that the proper choice of sodium compound and its concentration is critical for enhancing catalyst performance in FTS. The studied Co-Fe-based catalyst modified with 1% Na shows strong potential for efficient hydrocarbon production from syngas.
Keywords: Fischer–Tropsch synthesis, Syngas, High-molecular hydrocarbons, Cobalt–iron catalyst, Sodium promoter, Selectivity, Activity, α-value
Cite this paper: Asliddin Mamatov, Hayitali Ibodullayev, Normurot Fayzullaev, The Effect of Various Sodium Compounds and Their Concentrations on the Synthesis of High-Molecular Hydrocarbons from CO and H₂, International Journal of Materials and Chemistry, Vol. 15 No. 3, 2025, pp. 25-34. doi: 10.5923/j.ijmc.20251503.01.
Article Outline
1. Introduction
- In the 21st century, the sharp increase in global demand for energy resources—particularly in the industrial and transportation sectors—has led to significant economic and environmental challenges [1]. As a result, the development of environmentally friendly and efficient alternative technologies for the production of liquid fuels has become both a critical and urgent objective. Among such technologies, Fischer–Tropsch synthesis (FTS)-based processes such as gas-to-liquid (GTL), coal-to-liquid (CTL), and biomass-to-liquid (BTL) are of special importance. These routes offer great potential to ensure sustainable energy supply and global energy security, especially under conditions of declining conventional oil reserves.Fischer–Tropsch synthesis is a catalytic process in which synthesis gas (a mixture of CO and H₂) is converted into liquid hydrocarbons [2,3]. Although this technology was first developed nearly a century ago, it has recently gained renewed interest as a clean method for producing sulfur-free transportation fuels. Catalysts play a central role in the FTS process. The nature, composition, and physicochemical properties of the catalyst, along with operational parameters such as temperature, pressure, and H₂/CO molar ratio, strongly influence both the efficiency of the process and the distribution of the products.Currently, industrial and laboratory-scale studies widely employ catalysts based on ruthenium, nickel, cobalt, and iron. Among these, cobalt-based catalysts are distinguished by their high activity, strong selectivity towards long-chain paraffins, and low activity in the water-gas shift (WGS) reaction [4-6]. Several support materials have been investigated for cobalt-based catalysts, with Al₂O₃, SiO₂, and TiO₂ being the most common [7,8]. However, cobalt catalysts supported on Al₂O₃ often suffer from reduced reducibility due to strong metal-support interactions with cobalt oxides [9-11].To overcome this limitation and improve catalyst performance, promoters such as Pt, Pd, Re, and Ru are often introduced. These elements facilitate the reduction of cobalt oxides and enhance the activity of metallic cobalt [12,13]. In particular, Pd has been shown to effectively improve adsorption properties and increase hydrogenation reaction rates in cobalt catalysts [14-16], contributing to the development of highly active and stable catalytic systems.The key reactions in FTS involve the formation of long-chain paraffins and olefins. These can be represented as follows [17-21]:1. Paraffin synthesis:
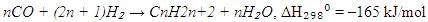 2. Olefin synthesis:
2. Olefin synthesis: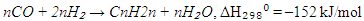 When the H₂/CO molar ratio is high, or when using catalysts with strong hydrogenation activity (e.g., cobalt or nickel), paraffin synthesis (reaction 1) predominates. In contrast, lower H₂/CO ratios or the use of iron-based catalysts favour olefin formation (reaction 2). In addition to the main reactions, several side reactions may occur during FTS, including:• Methanation:
When the H₂/CO molar ratio is high, or when using catalysts with strong hydrogenation activity (e.g., cobalt or nickel), paraffin synthesis (reaction 1) predominates. In contrast, lower H₂/CO ratios or the use of iron-based catalysts favour olefin formation (reaction 2). In addition to the main reactions, several side reactions may occur during FTS, including:• Methanation: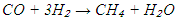 • Oxygenate formation:
• Oxygenate formation: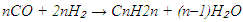 • CO₂ formation:
• CO₂ formation: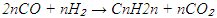 Among all catalyst systems, iron-based catalysts are notable for their high hydrogenation activity and tendency to promote CO₂ formation. To enhance their performance, promoters such as ThO₂ and K₂O are often added [22–27], which help maintain the integrity of active sites and improve thermal stability.Common support materials for FTS catalysts include Al₂O₃, SiO₂, TiO₂, polystyrene, and high-silica zeolites. In particular, high-silica zeolites (HSZs) improve process efficiency due to their superior thermal stability and acidic properties.While numerous studies have examined the role of alkali promoters in FTS, most have focused on potassium or rubidium. This study presents a novel comparative evaluation of five sodium compounds—NaNO₃, Na₂CO₃, NaOH, NaCl, and NaHCO₃—at varying concentrations on a Co–Fe–B–Zr-based catalyst supported on γ-Al₂O₃. Unlike previous research, our work systematically explores the interplay between sodium species, concentration, and chain growth (α-values), thereby filling a critical knowledge gap in optimizing long-chain hydrocarbon selectivity.
Among all catalyst systems, iron-based catalysts are notable for their high hydrogenation activity and tendency to promote CO₂ formation. To enhance their performance, promoters such as ThO₂ and K₂O are often added [22–27], which help maintain the integrity of active sites and improve thermal stability.Common support materials for FTS catalysts include Al₂O₃, SiO₂, TiO₂, polystyrene, and high-silica zeolites. In particular, high-silica zeolites (HSZs) improve process efficiency due to their superior thermal stability and acidic properties.While numerous studies have examined the role of alkali promoters in FTS, most have focused on potassium or rubidium. This study presents a novel comparative evaluation of five sodium compounds—NaNO₃, Na₂CO₃, NaOH, NaCl, and NaHCO₃—at varying concentrations on a Co–Fe–B–Zr-based catalyst supported on γ-Al₂O₃. Unlike previous research, our work systematically explores the interplay between sodium species, concentration, and chain growth (α-values), thereby filling a critical knowledge gap in optimizing long-chain hydrocarbon selectivity.2. Materials and Methods
2.1. Reaction Setup and Process Scheme
- The catalytic reactions were carried out using a high-pressure, fixed-bed flow-type laboratory reactor. This reactor, designed as an integral flow system, features a stationary catalyst bed and is capable of operating under pressures up to 10 atm. The technological configuration of the setup (Figure 1) is tailored for hydrocarbon synthesis experiments using synthesis gas (CO + H₂) under modern laboratory conditions.
 | Figure 1. Schematic diagram of a high-pressure fusion laboratory device |
2.2. Catalyst Preparation Method ge Style
- Catalysts with the composition 20%Co–20%Fe–5%B–1.5%Zr(0–2)%Na/Al₂O₃, aimed at synthesising high-molecular-weight hydrocarbons, were prepared via the incipient wetness impregnation method. In this process, aqueous solutions of cobalt(II) nitrate hexahydrate [Co(NO₃)₂·6H₂O], iron(III) nitrate [Fe(NO₃)₃·9H₂O], and zirconyl nitrate [ZrO(NO₃)₂·4H₂O] were impregnated onto a γ-Al₂O₃ support.After impregnation, the support was kept in a static state for 15 minutes to allow the solution to uniformly penetrate the pores and capillaries of the support. Subsequently, drying was carried out in a water bath at 60–65°C, promoting water evaporation and deposition of active components on the support surface. The dried samples were then calcined at 400°C for 1 hour to convert nitrate salts into metal oxides, forming catalytically active phases.
2.3. Catalyst Activation
- Before initiating the synthesis reaction, the catalysts were activated under reducing conditions to achieve high catalytic activity, selectivity, and productivity. In this stage, cobalt, iron, and other metal oxides were reduced to their active metallic forms, which generate catalytically active sites.Reduction was performed in a hydrogen flow at 400°C and a gas hourly space velocity of 3000 h⁻¹. The temperature was maintained for 1 hour to ensure complete reduction of the metal oxides and formation of the metallic phases (Co⁰, Fe⁰), which directly influence the efficiency of the FTS reaction.
2.4. Product Analysis Method
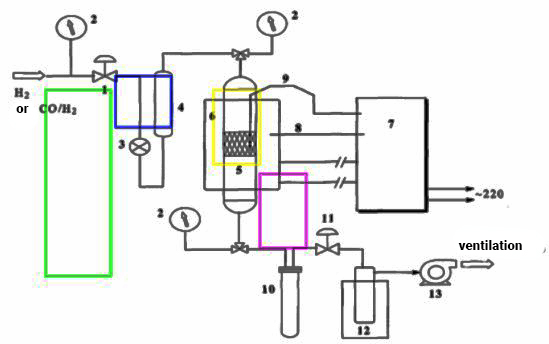
- The gaseous products of the reaction were analysed using a model LHM-80 gas chromatograph, which enables high-precision chromatographic analysis. Helium was used as the carrier gas due to its inertness and efficiency in providing a stable analytical environment. Two different chromatographic columns were employed:- First column: 1.5 m length, 3 mm internal diameter, packed with molecular sieve. It was used for analysing methane (CH₄) and carbon monoxide (CO).- Second column: 3 m length, 3 mm internal diameter. It was used for analysing carbon dioxide (CO₂) and C₂–C₄ hydrocarbons.Before gas analysis, argon was passed through both columns to purge the system and calibrate the chromatograph.
2.5. Chromatogram Interpretation
- The analysis results were obtained in the form of chromatograms (Figure 2), where each gas component was represented by a distinct peak corresponding to its retention time and concentration.
 | Figure 2. Chromatogram of gaseous products of synthesis |
3. Results and Discussion
3.1. Effect of Various Sodium Compounds on Catalyst Activity, Selectivity, and Liquid Product Composition
- The influence of different sodium precursors (NaNO₃, Na₂CO₃, NaOH, NaCl) on the catalytic performance of 20%Co–20%Fe–5%B–1.5%Zr/Al₂O₃ catalysts was systematically investigated in the Fischer–Tropsch synthesis (FTS) process. The sodium content in all tested samples was fixed at 1 mol%. The analysis focused on CO conversion, C₅⁺ product selectivity, and overall hydrocarbon productivity.
3.2. Catalyst Modified with Na₂CO₃
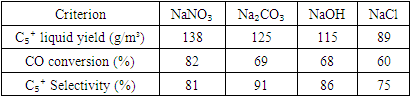
- When Na₂CO₃ was used as the sodium source, the yield of liquid hydrocarbons increased from 115 g/m³ to 125 g/m³. Simultaneously, the yield of gaseous C₁–C₄ hydrocarbons decreased from 16 g/m³ to 12 g/m³, indicating improved selectivity toward higher molecular weight hydrocarbons. The CO conversion reached 69%, reflecting efficient CO adsorption and activation on the catalyst surface. The selectivity toward C₅⁺ fractions increased from 86% to 91%, suggesting that the catalyst promotes chain-growth reactions, favouring the formation of long-chain paraffins. The methane selectivity was low (4%), indicating suppression of the methanation side reaction.Compared to the study by Wang et al. [21], where a C₅⁺ selectivity of 83% was achieved using Na₂CO₃-modified Fe catalysts, our bimetallic Co–Fe catalyst system exhibited higher selectivity (91%) and a lower methane selectivity (4%). This suggests that the synergy between cobalt and iron, along with Zr promotion, contributes to enhanced chain growth reactions.
3.3. Catalyst Modified with NaOH
- The catalyst prepared with NaOH exhibited a CO conversion of 68% and a C₅⁺ hydrocarbon productivity of 115 g/m³. Notably, the Anderson–Schulz–Flory (ASF) chain growth probability (α) was 0.89, implying a high potential for producing longer hydrocarbon chains. The C₁₉⁺ fraction accounted for 19% of the liquid products, suggesting an enrichment of heavy fractions suitable for diesel and wax applications.According to Zhao et al. [23], the use of NaOH in Fe-based catalysts increased the α-value up to 0.86. In our study, this parameter reached 0.89 with the Co–Fe system, indicating an even stronger tendency toward long-chain hydrocarbon formation, likely due to enhanced electron donation and catalyst basicity.
3.4. Compositional Characteristics of Synthesised Products
- The liquid hydrocarbon products obtained from all catalyst series were predominantly paraffinic in nature, accounting for 94–96% of the total composition. Among these, linear alkanes constituted 65–80% of the products. The predominance of linear structures is indicative of a highly selective reaction pathway enabled by the optimised catalyst surface properties.
3.5. Comparative Efficiency of Sodium Compounds
- Table 1 summarises the catalytic performance metrics for each sodium compound used in the modification process.
|
3.6. Influence of Sodium Compound and Its Concentration
- The nature and concentration of sodium additives significantly influenced the catalyst performance in FTS. The catalyst modified with NaNO₃ demonstrated superior activity and productivity in tests conducted at 210°C. Specifically:• CO conversion reached 82%,• C₅⁺ liquid hydrocarbon yield was 138 g/m³,• Selectivity toward C₅⁺ products was 81%.These results confirm that NaNO₃ efficiently restructures active catalytic centres, thereby directing the reaction toward the formation of higher molecular weight products.Moreover, increasing the Na⁺ ion concentration from 1% to 2% led to:• An increase in CO conversion from 82% to 83%,• An increase in C₅⁺ productivity from 127 g/m³ to 135 g/m³,• A decrease in selectivity toward C₅⁺ products from 92% to 84%.Simultaneously, the selectivity toward light gaseous products (C₁–C₄) increased from 7% to 12%, implying that excess sodium promotes methane formation and short-chain hydrocarbon generation.A study by Kim et al. [27] also identified 1 mol% Na as the optimal concentration for alkali-promoted Co-based FTS catalysts, reporting similar selectivity trends. Higher Na levels were associated with increased formation of gaseous by-products, consistent with our findings.
3.7. Summary of Trends and Observations
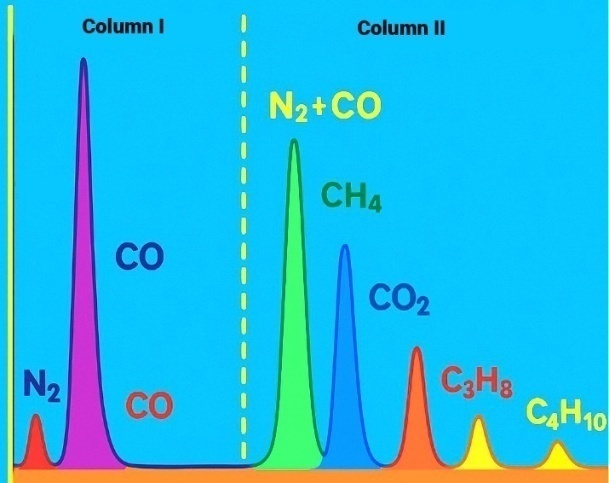
- The type of sodium compound plays a crucial role in modifying the electronic environment and active sites of the catalyst. Specifically:• NaNO₃ yielded the best results in terms of activity and productivity,• Na₂CO₃ favoured selectivity toward desired heavy products,• NaOH provided optimal chain growth (high α),• NaCl resulted in diminished catalytic efficiency.Additionally, sodium concentration exhibited a nonlinear effect. An optimal level around 1 mol% was found to provide the best balance between activity, selectivity, and yield. Increasing the amount beyond this level reduced selectivity due to excessive formation of gaseous byproducts.Figure 3 illustrates the trends in CO conversion (XCO), C₅⁺ selectivity (SC₅⁺), and C₁–C₄ gas selectivity (SC₁–C₄) as a function of sodium content. At 1 mol% Na, the maximum C₅⁺ selectivity (92%) and minimum gas yield (7%) were observed. In contrast, the unpromoted catalyst (0% Na) showed high CO conversion (94%) but poor selectivity (SC₅⁺ = 41%) with high gas formation (44%). At 2–5 mol% Na, conversion remained relatively high, but a drop in selectivity was evident.
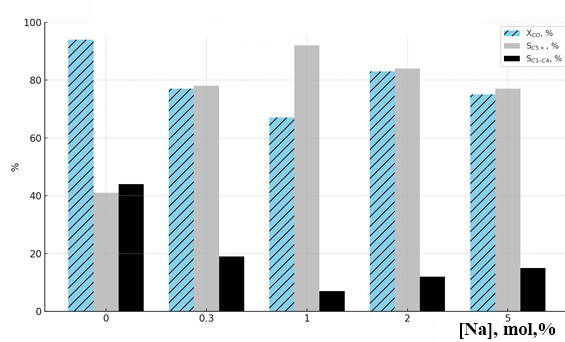 | Figure 3. Effect of sodium content in the 20%Co–20%Fe–5%B–1.5%Zr(0–2)%Na/Al₂O₃ catalyst composition on catalytic properties in the synthesis of hydrocarbons from CO and H₂ at T = 200°C |
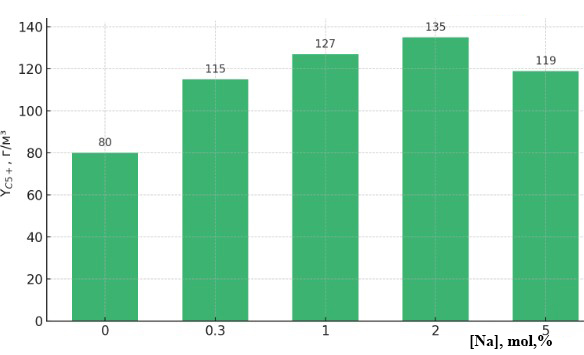 | Figure 4. Effect of sodium content in 20%Co–20%Fe–5%B–1.5%Zr(0–5)%Na/Al₂O₃ catalyst composition on the yield of liquid C₅⁺ hydrocarbons at T = 200°C |
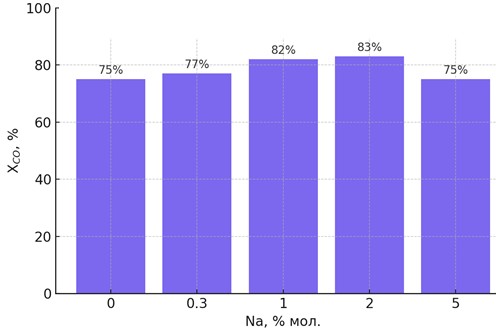 | Figure 5. A diagram showing the effect of Na concentration on CO conversion |
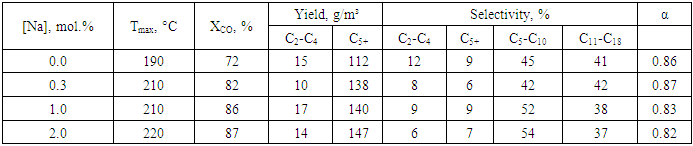
3.8. Comparative Analysis of Sodium Concentration Effects
- When analyzing the results obtained by varying sodium concentration from 0% to 5% in catalysts selected for Fischer–Tropsch synthesis, the most optimal combination for hydrocarbon synthesis was observed in the catalyst 20%Co–20%Fe–5%B–1.5%Zr(0–2)%Na/Al₂O₃. In this case:• CO conversion (XCO) reached 82%;• C₅⁺ fraction yield increased to 138 g/m³;• Selectivity for liquid fractions (SC₅⁺) was recorded at 81%.Such high efficiency is attributed to the optimal amount of Na⁺ ions introduced on the catalyst surface, leading to proper formation of active centres, improved metal dispersion, and more effective adsorption of gas components.The results in Table 2 show that both excessive and insufficient Na⁺ concentrations negatively affect reaction efficiency:• At 0.3% Na⁺, a good balance between selectivity and productivity is achieved;• At 1–2% Na⁺, maximum CO conversion and C₅⁺ fraction yield are obtained;• The methanation reaction remains suppressed at a low level.
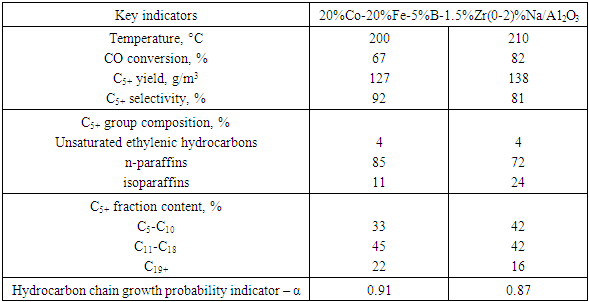
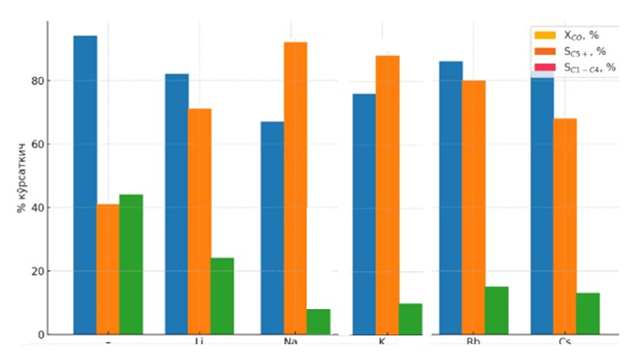 | Figure 6. Effect of Group I alkali metals (M = Li, Na, K, Rb, Cs) on hydrocarbon synthesis from CO and H₂ at T = 200°C using the 20%Co–20%Fe–5%B–1.5%Zr-1M/Al₂O₃ catalyst |
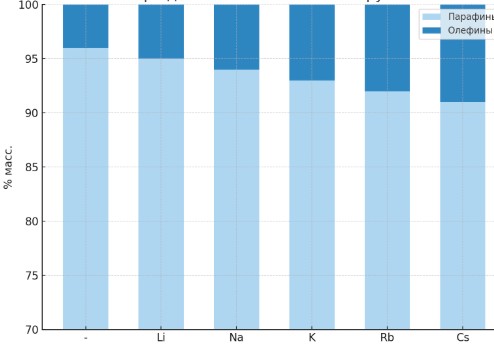 | Figure 7. Variation in the content of paraffins and unsaturated hydrocarbons (olefins) in the reaction products over catalysts with the composition 20%Co–20%Fe–5%B–1.5%Zr–1M/Al₂O₃ (where M = Li, Na, K, Rb, Cs). This figure illustrates the influence of Group I alkali metal elements, introduced as modifiers into the catalyst composition, on the product selectivity of the Fischer–Tropsch synthesis process |
|
4. Conclusions
- 1. In the catalysts with the composition 20%Co–20%Fe–5%B–1.5%Zr–1M/Al₂O₃, where M = Li, Na, K, Rb, Cs, the formation process of aliphatic hydrocarbons from synthesis gas (CO + H₂) was systematically studied. Elements of Group I of the periodic table were introduced into the catalyst composition as modifying additives, and the nature of these elements significantly affected the efficiency of the reaction. This study made it possible to determine how the composition of the hydrocarbon fractions, selectivity, and the length of the hydrocarbon chain change under the influence of the modifier elements.2. In the recommended catalysts with the composition 20%Co–20%Fe–5%B–1.5%Zr(x%Na)/Al₂O₃ used for the synthesis process, an increase in the amount of strongly bound CO was observed. This factor is considered one of the important elements that ensure maximum selectivity in the formation of liquid hydrocarbons. In other words, the long-term retention of CO molecules in an active adsorbed state on the catalyst surface enhances the efficiency of the chain-growth mechanism, leading to a predominant formation of C₅⁺ hydrocarbon fractions.3. The nature of the sodium precursor compound used in the catalyst modification process also has a significant effect on the efficiency of the reaction. The effectiveness of hydrocarbon formation, particularly the yield of C₅⁺ fractions (YC₅⁺), was found to decrease in the following order:NaNO₃ (138 g/m³) > Na₂CO₃ (125 g/m³) > NaOH (115 g/m³) > NaCl (89 g/m³)These values are associated with the nature of the anion in the sodium compounds, their hygroscopicity, and degree of ionisation, which influence their interaction with the active sites of the catalyst. The activity and reactivity of the NO₃⁻ ion increase the activity of the catalyst surface, thereby contributing to higher selectivity. Despite the promising results, this study has several limitations. First, the experiments were conducted under fixed temperature and pressure conditions, limiting the generalizability to other industrial settings. Second, long-term catalyst stability and regeneration performance were not assessed. Further investigations using continuous flow reactors and extended operation time are recommended. Our results align with the findings of Ahmed and coworkers, who observed that Na and Cs significantly enhance the α-value and C₅⁺ selectivity in Co-based catalysts. The strong electron-donating capacity and large ionic radius of these elements were suggested to play a key role in stabilising long-chain intermediates.
Future Research Directions
- Future studies should explore the effect of bimetallic promoter systems (e.g., Na–K, Na–Cs) and their synergistic interactions. Investigating the use of mesoporous or hierarchical supports may also provide further improvements in product selectivity and catalyst lifetime. Additionally, mechanistic studies using in-situ spectroscopic techniques (e.g., DRIFTS, XPS) are essential to elucidate the role of sodium species during chain growth.
References
| [1] | S. Mehariya, A.K. Patel, P.K. Obulisamy, E. Punniyakotti, J.W.C. Wong, Codigestion of food waste and sewage sludge for methane production: current status and perspective, Bioresour. Technol. 265 (2018) 519531. 2. |
| [2] | O. Parthiba Karthikeyan, E. Trably, S. Mehariya, N. Bernet, J.W.C. Wong, H. Carrere, Pretreatment of food waste for methane and hydrogen recovery: a review, Bioresour. Technol. 249 (2018) 10251039. |
| [3] | A.S. Snehesh, H.S. Mukunda, S. Mahapatra, S. Dasappa, Fischer-Tropsch route for the conversion of biomass to liquid fuels—technical and economic analysis, Energy 130 (2017) 182191. |
| [4] | W. Chen, T. Lin, Y. Dai, Y. An, F. Yu, L. Zhong, et al., Recent advances in the investigation of nanoeffects of Fischer-Tropsch catalysts, Catal. Today 311 (2018) 822. |
| [5] | N. Moazami, M.L. Wyszynski, K. Rahbar, A. Tsolakis, Parametric study and multiobjective optimisation of fixed-bed Fischer-Tropsch (FT) reactor: the improvement of FT synthesis product formation and synthetic conversion, Ind. Eng. Chem. Res. 56 (2017) 94469466. |
| [6] | M. Rafati, L. Wang, D.C. Dayton, K. Schimmel, V. Kabadi, A. Shahbazi, Technoeconomic analysis of production of Fischer-Tropsch liquids via biomass gasification: the effects of Fischer-Tropsch catalysts and natural gas co-feeding, Energy Convers. Manage. 133 (2017) 153166. |
| [7] | Y. Xue, H. Ge, Z. Chen, Y. Zhai, J. Zhang, J. Sun, et al., Effect of strain on the performance of iron-based catalyst in Fischer-Tropsch synthesis, J. Catal. 358 (2018) 237242. |
| [8] | H. Mahmoudi, M. Mahmoudi, O. Doustdar, H. Jahangiri, A. Tsolakis, S. Gu, et al., A review of Fischer Tropsch synthesis process, mechanism, surface chemistry and catalyst formulation, Biofuels Eng. 2 (2017) 1131. 240 Lignocellulosic Biomass to Liquid Biofuels. |
| [9] | A. Chiodini, L. Bua, L. Carnelli, R. Zwart, B. Vreugdenhil, M. Vocciante, Enhancements in biomass-to-liquid processes: gasification aiming at high hydrogen/ carbon monoxide ratios for direct Fischer-Tropsch synthesis applications, Biomass Bioenergy 106 (2017) 104114. |
| [10] | Y. Xu, D. Liu, X. Liu, Conversion of syngas toward aromatics over hybrid Fe-based Fischer-Tropsch catalysts and HZSM-5 zeolites, Appl. Catal.., A: Gen. 552 (2018) 168183. |
| [11] | E. Garcia-Bordeje, Y. Liu, D.S. Su, C. Pham-Huu, Hierarchically structured reactors containing nanocarbons for intensification of chemical reactions, J. Mater. Chem. A 5 (2017) 2240822441. |
| [12] | M.G.A. Cruz, A.P.S. de Oliveira, F.A.N. Fernandes, F.F. de Sousa, A.C. Oliveira, J. M. Filho, et al., Fe-containing carbon obtained from ferrocene: influence of the preparation procedure on the catalytic performance in FTS reaction, Chem. Eng. J. 317 (2017) 143156. |
| [13] | M.G.A. Cruz, F.A.N. Fernandes, A.C. Oliveira, J.M. Filho, A.C. Oliveira, A.F. Campos, et al., Effect of the calcination temperatures of the Fe-based catalysts supported on polystyrene mesoporous carbon for FTS synthesis, Catal. Today 282 (2017) 174184. |
| [14] | X. Wei, H. Wang, Z. Yin, S. Qaseem, J. Li, Tubular electrocatalytic membrane reactor for alcohol oxidation: CFD simulation and experiment, Chin. J. Chem. Eng. 25 (2017) 1825. |
| [15] | V.S. Ermolaev, K.O. Gryaznov, E.B. Mitberg, V.Z. Mordkovich, V.F. Tretyakov, Laboratory and pilot plant fixed-bed reactors for Fischer Tropsch synthesis: mathematical modeling and experimental investigation, Chem. Eng. Sci. 138 (2015). |
| [16] | Y. Sun, J. Wei, J.P. Zhang, G. Yang, Optimization using response surface methodology and kinetic study of FischerTropsch synthesis using SiO2 supported bimetallic CoNi catalyst, J. Nat. Gas Sci. Eng. 28 (2016) 173183. |
| [17] | N. Moazami, M.L. Wyszynski, K. Rahbar, A. Tsolakis, H. Mahmoudi, A comprehensive study of kinetics mechanism of Fischer-Tropsch synthesis over cobalt-based catalyst, Chem. Eng. Sci. 171 (2017) 3260. |
| [18] | V. Vosoughi, S. Badoga, A.K. Dalai, N. Abatzoglou, Modification of mesoporous alumina as a support for cobalt-based catalyst in Fischer-Tropsch synthesis, Fuel Process. Technol. 162 (2017) 5565. |
| [19] | Savost’yanov, R.E. Yakovenko, S.I. Sulima, V.G. Bakun, G.B. Narochnyi, V. M. Chernyshev, et al., The impact of Al2O3 promoter on an efficiency of C5 hydrocarbons formation over Co/SiO2 catalysts via Fischer-Tropsch synthesis, Catal. Today 279 (2017) 107114. |
| [20] | Li, Z.L.; Wang, J.J.; Qu, Y.Z.; Liu, H.L.; Tang, C.Z.; Miao, S.; Feng, Z.C.; An, H.Y.; Li, C. Highly selective conversion of carbon dioxide to lower olefins. ACS Catal. 2017, 12, 8544–8548. [CrossRef] |
| [21] | Guo, L.S.; Sun, J.; Ji, X.W.; Wei, J.; Wen, Z.Y.; Yao, R.W.; Xu, H.Y.; Ge, Q.J. Directly converting carbon dioxide to linear α-olefins on bio-promoted catalysts. Commun. Chem. 2018, 1, 11. [CrossRef] |
| [22] | Lu, P.F.; Liang, J.; Wang, K.Z.; Liu, B.; Atchimarungsri, T.; Wang, Y.; Zhang, X.J.; Tian, J.M.; Jiang, Y.J.; Liu, Z.H.; et al. Boosting liquid hydrocarbons synthesis from CO2 hydrogenation via tailoring acid properties of HZSM-5 zeolite. Ind. Eng. Chem. Res. 2022, 61, 16393–16401. [CrossRef] |
| [23] | Jiang, B.; Xia, D.; Yu, B.; Xiong, R.; Ao, W.; Zhang, P.; Cong, L. An environment-friendly process for limestone calcination with CO2 looping and recovery. J. Clean. Prod. 2019, 240, 118147. |
| [24] | Numpilat, T.; Chanlek, N.; Poo-arporn, Y.; Cheng, C.K.; Siri-Nguan, N.; Sornchamni, T.; Witoon, T. Tuning interactions of surface-adsorbed species over Fe-Co/K-Al2O3 catalyst by different K contents: Selective CO2 hydrogenation to light olefins. ChemCatChem 2020, 12, 3306–3320. |
| [25] | Guo, L.S.; Sun, J.; Wei, J.; Wen, Z.Y.; Xu, H.Y.; Ge, Q.J. Fischer–Tropsch synthesis over iron catalysts with corncob-derived promoters. J. Energy Chem. 2017, 26, 632–638. [CrossRef] |
| [26] | Gao, X.H.; Zhang, J.L.; Chen, N.; Ma, Q.X.; Fan, S.B.; Zhao, T.S.; Tsubaki, N. Effects of zinc on Fe-based catalysts during the synthesis of light olefins from the Fischer-Tropsch process. Chin. J. Catal. 2016, 37, 510–516. |
| [27] | Gnanamani, M.K.; Jacobs, G.; Shafer, W.D.; Martinelli, M.; Cronauer, D.C.; Kropf, A.J.; Marshall, C.L.; Davis, B.H. Ga and In modified ceria as supports for cobalt-catalyzed Fischer-Tropsch synthesis. Appl. Catal. A 2017, 547, 115–123. |
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML