-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
International Journal of Materials and Chemistry
p-ISSN: 2166-5346 e-ISSN: 2166-5354
2025; 15(2): 15-19
doi:10.5923/j.ijmc.20251502.01
Received: May 6, 2025; Accepted: Jun. 2, 2025; Published: Jun. 17, 2025

Synthesis of Multi-Component Coded Quantum Dots
Ishankulov A. F.1, Islomova Z. R.1, Tursunova N. R.1, Khalilov K. F.1, Mukhamadiev N. K.1, Galyametdinov Y. G.2
1Samarkand State University, Samarkand, Uzbekistan
2Kazan National Research Technological University, Kazan, Russia
Correspondence to: Ishankulov A. F., Samarkand State University, Samarkand, Uzbekistan.
| Email: |  |
Copyright © 2025 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

In this study, a polymethyl methacrylate (PMMA) polymer doped with fluorescent nanocrystals was obtained based for the identification of petroleum products. The components were mixed in a matrix of optically transparent PMMA polymer. Colloidally produced CdSe, CdSe and CdSe/ZnS quantum dots were stabilized with oleic acid and their optical properties were studied. It was observed that the intensity peaks of the quantum dots did not change after modification with PMMA polymer. The concentration of two different quantum dots was controlled by doping. Such microspheres allow to increase the efficiency of diagnostics of flows in wells during development of oil and gas fields. These obtained markers serve to prevent counterfeiting of petroleum products.
Keywords: Polymer, Luminescence, Triplet, CdSe/CdS/ZnS, Nanoparticles, Oil, Synthesis, Absorption, Nanoparticle, Photoluminescence
Cite this paper: Ishankulov A. F., Islomova Z. R., Tursunova N. R., Khalilov K. F., Mukhamadiev N. K., Galyametdinov Y. G., Synthesis of Multi-Component Coded Quantum Dots, International Journal of Materials and Chemistry, Vol. 15 No. 2, 2025, pp. 15-19. doi: 10.5923/j.ijmc.20251502.01.
Article Outline
1. Introduction
- Quantum dots (QDs) are small semiconductor crystals with a size of 2-10 nanometers, containing hundreds to several thousand atoms. QDs are attracting the interest of researchers as a new generation of semiconducting inorganic crystals. They are technically defined as "small crystals containing a variable number of electrons that occupy well-defined discrete quantum states and have electronic properties intermediate between discrete fundamental particles" [1-3].These are nano-sized semiconductor nanoparticles with fluorescent characteristics whose optical properties vary depending on their chemical composition, size, dispersion and shape. The growing interest in quantum dots is due to their superior photostability compared to conventional dyes due to, narrow and intense size-dependent luminescence, and broad light absorption [4–6].In this work, CdSe/ZnS and CdS/ZnS nanocrystals with high luminescence intensity were synthesized and embedded into polymethyl methacrylate beads. Incorporating quantum dots into monodisperse polymer microspheres can yield materials with reproducible properties that can be used practically in applications such as optical coding, biological assays, optical data storage, and sensing.In addition to serving as a matrix for the polymer quantum dot material, it also contributes to the mechanical and chemical stability of the material. This increases interest in the use of quantum dots in the polymer matrix of different types of fluorescent materials. In addition, polymers have the potential to process nanocomposites into technologically important structures such as thin films to prevent adhesion. Despite the fact that nanocomposites based on polymer and quantum dots have been used in various fields for many years, the mixing of these two composites leads to a destruction of the optical and electrical properties of nanocomposites. In order to prevent this, the inclusion of QDs in the polymer matrix was achieved to avoid these shortcomings [7-11].Currently, the main approach to introducing quantum dots into polymer materials is to co-disperse nanoparticles and other substances (liquid crystals, coordination compounds) in a solvent and then remove it [12]. However, this method often leads to the formation of aggregates of nanoparticles distributed unevenly throughout the volume [13-15]. The distribution of nanoparticles in the solution matrix, as well. In addition, there are studies on the use of thiol-containing substances as stabilizers in the synthesis of quantum dots [16]. It was found that other methods can be used, which rely on the direct coating of QDs with amphiphilic polymers rather than the creation of polymer micelles. Amphiphilic and alkyl-modified low molecular weight polyacrylic acids can coat trioctylphosphinoxide-protected nanocrystals and make quantum dots (QDs) soluble in water [17].
2. Experimental
- Materials and reagentsThe following reagents were used in the experimental part of the study: Cadmium precursor (CdO, 98%, Sigma Aldrich, USA), selenium precursor (Se, 99%, Vekton, Russia), sulfur powder (S, 99.99%, Lenreaktiv, Russia), zinc precursor (ZnCl2, 99%, Reahim, Russia), dodecanethiol (DDT, Sigma Aldrich, USA), rhodamine 6G (96%, Ecos, Russia), oleic acid (90%, Ecos, Russia), 1-octadecene (ODE, 90%, Acros, Belgium), solvents: toluene (99%, Vekton, Russia), ethanol (96%, Ekos, Russia), acetone (99%, Ekos, Russia), chloroform (Sigma Aldrich, USA). All reagents were used without additives.Absorption spectra were studied in this study on a Perkin Elmer Instrumental LAMBDA 35 UV/VIS (ultraviolet–visible) spectrometer. Luminescence spectra were recorded on a computer-controlled Varian Cary Eclipse spectrofluorimeter. Measurements were made at room temperature, i.e. 25°C.The hydrodynamic sizes of CdSe/ZnS and CdSe/ZnS/ZnS QDs coated with oleic acid were determined by dynamic light scattering on a Malvern Zetasizer Nano photon correlation spectrometer.Synthesis of "core", "core/shell" and "core/shell/shell" structural nanoparticlesFirst, the cadmium solution was prepared as follows: after CdO was dissolved in oleic acid at a temperature of 220°C until the solution became clear, selenium powder was dissolved in trioctylphosphine to a temperature of 180°C, the synthesis temperature was heated to a temperature of 240°C, and the selenium solution was mixed into the CdO solution for 15 minutes. In order to remove impurities from the obtained CdSe quantum dots, solvents toluene and ethanol were mixed in a ratio of 1:1, CdSe nanoparticles were mixed and centrifuged at a speed of 5000 for 15 minutes.To obtain hybrid CdSe/ZnS quantum dots, 1 ml of synthesized and purified CdSe nanoparticles were taken, heated to a temperature of 200°C in TOP, zinc and sulfur solutions were prepared [18], Zn and S solutions were slowly mixed and added to the reaction mixture. The formation of a ZnS QD layer occurs after adding a sulfur solution containing the zinc precursor octadecene to the reaction mixture at 240°C for 15 min in the presence of dodecanethiol.After synthesis, all resulting nanoparticles were purified by triple precipitation in a toluene/ethanol mixture and dissolved in chloroform for spectroscopic studies.The synthesis of CdSe/ZnS/ZnS quantum dots with a triplet core/shell/shell structure requires two steps of coating with ZnS shells. In this case, CdSe/ZnS nanoparticles are synthesized using the method described above [19]. After that, a solution of "cations" was obtained by mixing 0.02 g of cadmium acetate dihydrate and 0.44 g of zinc acetate dihydrate in 5 ml of oleic acid at 120°C until complete dissolution. Meanwhile, a solution of "anions" was obtained by dissolving 6 mg of selenium and 28 mg of elemental sulfur in a mixture of 1 ml of trioctylphosphine and 4 ml of octadecene at 120°C. Then, the solutions of "cations" and "anions" were mixed and the temperature of the reaction mixture was raised to 300°C. The synthesis of nanoparticles was carried out under a nitrogen atmosphere at a certain temperature for 30 minutes. At the end of the reaction, the quantum dots were purified by triple precipitation from an ethanol/toluene mixture.Synthesis of PMMATo synthesize PMMA, 40 ml of a 2% aqueous solution of polyvinylpyrrolidone K25 was poured into a 100 ml three-neck round-bottom flask and dissolved in 100 mg of sodium persulfate as an initiator. A mixture of 4 ml of methyl methacrylate, 0.2 ml of acrylic acid, 0.2 ml of divinylbenzene was introduced into the aqueous solution using a high-pressure mechanical stirrer. Next, the mixture was heated to 65°C in an atmosphere of inert gas argon using a glycerin bath and a magnetic stirrer. Polymerization was carried out at 1200 rpm for 5 hours. The resulting polymer suspension was diluted another 2 times with distilled water, separated by centrifugation into 3 fractions and washed with distilled water. The first fraction was obtained at a speed of 2000 rpm, the second at a speed of 6000 rpm, and the third at a speed of more than 6000 rpm.Obtained quantum dots CdSe/ZnS (size 2.8 nm) and CdSe/CdS/ZnS (size 4.7 nm) mixed with PMMA in a ratio of 1:1, mixed with 3.6 ml of propyl alcohol and mixed for 3 hours using magnetic stirrer. For cleaning non-reactive raw materials, use a mixture of hexane and propanol in a ratio of 1:1. Then rinse with distilled water (5ml).The effective radius (R) was calculated from the diffusion coefficients using the Stokes-Einstein equation based on the first cumulant:
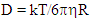 where k is the Boltzmann constant, T is the absolute temperature,
where k is the Boltzmann constant, T is the absolute temperature, 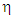 is the viscosity of the solvent.
is the viscosity of the solvent.3. Results and Discussion
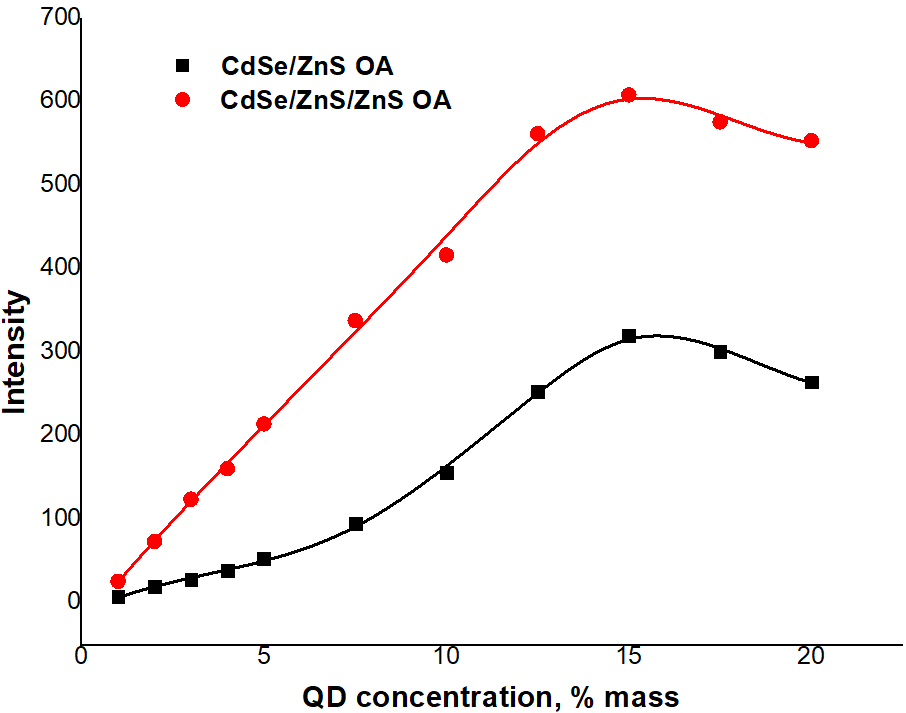
- As we see, in the absorption spectra there is a shift of exciton peaks to the long-wavelength (bathochromic) region, which indicates an increase in the size of nanoparticles (Fig. 1).
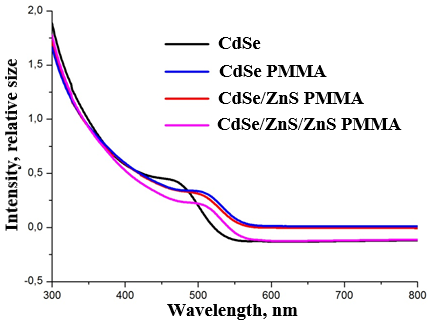 | Figure 1. Absorption spectra of CdSe, CdSe-PMMA, CdSe/ZnS-PMMA, and CdSe/ZnS/ZnS QDs |
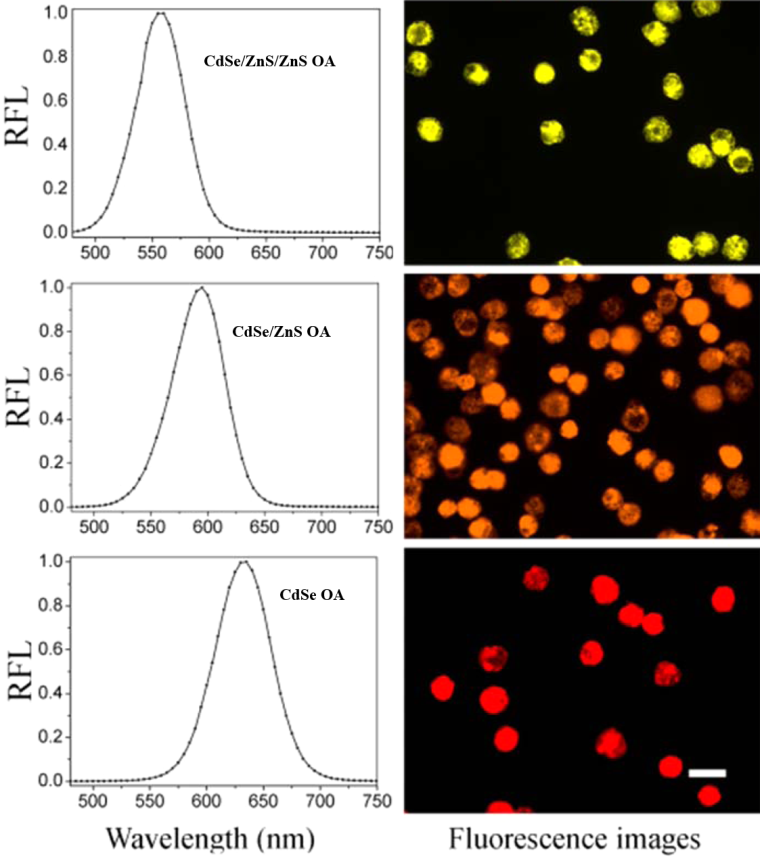 | Figure 2. Luminescence spectra of yellow (CdSe/ZnS/ZnS), orange (CdSe/ZnS) and red (CdSe) quantum dots and fluorescence images |
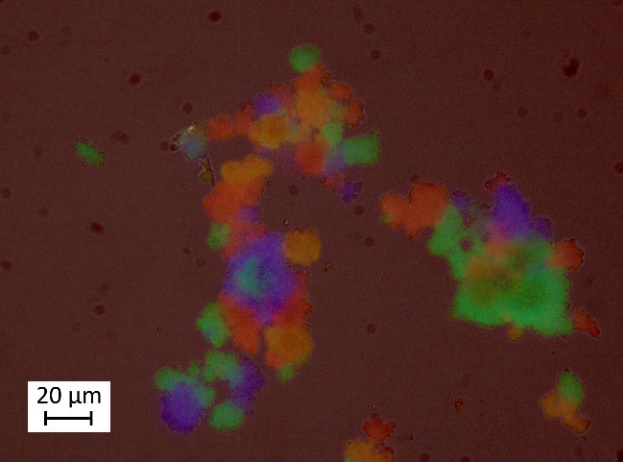 | Figure 3. Optical microscopy image of CdSe, CdSe/ZnS and CdSe/ZnS/ZnS quantum dots illuminated by laser light |
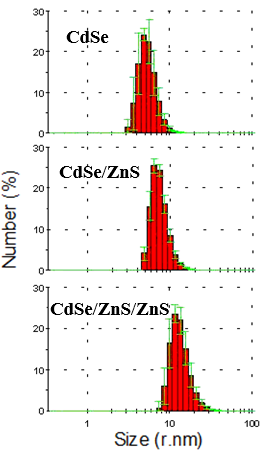 | Figure 5. Average hydrodynamic sizes of CdSe, hybrid CdSe/ZnS and triplet CdSe/ZnS/ZnS quantum dots |
4. Conclusions
- As part of this research, the colloidal method of nanoparticle synthesis was improved, as a result of which CdSe, hybrid CdSe/ZnS and triplet CdSe/ZnS quantum dots with improved luminescent properties appeared in the luminescence maximum wavelength range of 565-635 nm. After inserting the obtained quantum dots in the polymethyl methacrylate matrix, it was observed that the stability of composite materials increased. Such microspheres allow to increase the efficiency of diagnostics of flows in wells during development of oil and gas fields. These obtained markers serve to prevent counterfeiting of petroleum products.
Formatting of Funding Sources
- The author declare that he did not receive any grants for this research.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML