Nasiba Kambaralievna Sadikova1, Mukhtar Rakhmatovich Amonov2
1Independent Researcher, Bukhara State University, Bukhara, Uzbekistan
2Professor, Bukhara State University, Bukhara, Uzbekistan
Copyright © 2025 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Abstract
This article presents a novel coagulation–flocculation–adsorption method for the treatment of complex wastewater, which involves the stages of pH regulation, treatment with composite reagents, sediment separation, adsorption, and filtration. The method is shown to efficiently remove dyes, surfactants, and complex organic and inorganic pollutants with an efficiency of 97.0–98.7%. The study demonstrates that the use of composite reagents increases the sedimentation rate of coagulated and flocculated aggregates and improves the purification process compared to their aqueous analogs. Furthermore, this method simplifies the treatment process by reducing the number of technological stages and the amount of introduced coagulants and flocculants. New composite reagents containing powdery aluminum sulfate and iron chloride coagulants, polyacrylamide–based flocculants, and carboxymethyl cellulose (CMC) as a stabilizer have been developed. These reagents demonstrate effective application when the coagulant and flocculant mass ratio is 1.0:1.0, and when applied at a dosage of 2.0 g/L, they result in significant purification efficiency. The findings contribute to the advancement of wastewater treatment technologies in industries producing complex effluents, particularly the textile industry.
Keywords:
Coagulation, Flocculation, Adsorption, Wastewater treatment, Composite reagents, Carboxymethyl cellulose (CMC), Dyes removal, Surfactants, Textile wastewater, Environmental engineering, Pollutant removal, Sedimentation, Filtration, Influence, Chemical oxygen demand (COD)
Cite this paper: Nasiba Kambaralievna Sadikova, Mukhtar Rakhmatovich Amonov, Study of the Influence of Cleaning Component Concentration on the Efficiency of Wastewater Treatment, International Journal of Materials and Chemistry, Vol. 15 No. 1, 2025, pp. 9-14. doi: 10.5923/j.ijmc.20251501.03.
1. Introduction
In today’s world, the issue of water purification stands at the forefront of environmental and technological challenges. Fresh water resources are becoming increasingly scarce, while pollution of water systems from industrial, agricultural, and domestic discharges is leading to serious environmental consequences. The problem is relevant for most countries, including the Republic of Uzbekistan.In Uzbekistan, water resources are of vital importance for agriculture and for supplying the population with drinking water. However, the deterioration in the quality of water in the country’s rivers and lakes is a cause for serious concern. Industrial and agricultural discharges, as well as pollution from domestic and sewage effluents, contribute to the contamination of water resources.Given this situation, research in the field of water purification is becoming extremely important for the Republic of Uzbekistan. It is necessary to develop and implement effective and sustainable water treatment methods that can improve the quality of water resources and ensure the safety of water use for the population and agriculture.The use of natural and modified bentonite clays as adsorbents for removing contaminants from water represents one of the promising strategies. These materials have high adsorption capacity and can be effective in purifying water from various pollutants. Furthermore, the development and application of such methods can contribute to the sustainable management of water resources in Uzbekistan.The current state of water purification highlights the importance of developing effective methods and materials for removing contaminants from water. This is a pressing issue in today’s world, where maintaining the quality of water resources plays a key role in both human health and environmental protection. The structural characteristics of silicates–particularly layered silicates–make them potentially suitable for use as adsorbents. Their large surface area and potential for interlayer interactions enhance their adsorption properties.
2. The Main Findings and Results
The modification of natural layered silicates offers broad opportunities for improving their adsorption capabilities. Acid treatment, cation exchange, and chemical modification allow the creation of adsorbents with specific characteristics tailored to particular needs [1-5].It has been found that modified bentonites possess a high capacity for sorbing petroleum products. It was determined that when using AB (alkaline bentonite) and AEB (alkaline–earth bentonite), the removal efficiency of these substances does not exceed 60%. In contrast, TOAB (thermally enriched alkaline bentonite) and TOEAB (thermally enriched alkaline–earth bentonite) achieve purification levels of over 80%. Moreover, systems utilizing CMAB (chemically modified alkaline bentonite) and CMEAB (chemically modified alkaline–earth bentonite) are capable of oxidizing up to 99% of contaminants in just 20–25 minutes. It is recommended to use oxidative systems based on modified bentonites, such as CMAB and CMEAB.Based on the above, it became necessary to study the influence of the concentration and nature of various chemical reagents on the efficiency of wastewater treatment at oil refining facilities. We examined the effect of temperature on the performance of coagulants and flocculants in wastewater treatment. As an indicator of the effectiveness of coagulant and flocculant action–serving as the main criterion for the treatment of wastewater from oil refineries–we used the turbidity values of dispersions formed as a result of hydrolysis after 30 minutes of settling from the time the reagents were introduced, which reflects their sedimentation rate.The essence of the method for treating wastewater from various primary impurities is as follows: a measured volume of wastewater is treated with thermochemically modified bentonite as a sorbent, considering the pollution level, in an amount ranging from 3 to 5 mg/L with a particle size of 0.2–0.4 µm, and stirred for 3–5 minutes. After adding the coagulant and flocculant, the mixture is stirred again for 5 minutes. To increase the efficiency of purification and the rate of sedimentation, we introduced a 10% solution of slaked lime in the amount of 3–5 mL. The resulting suspension was allowed to settle for 10–15 minutes. For each sample of treated water, the effectiveness of the flocculant was evaluated, aiming to achieve the highest purification degree depending on the flocculant concentration. The degree of decolorization was determined using a photoelectric colorimeter (FEK–LF–72M).For each water sample, depending on turbidity, color, and pH of the wastewater, the appropriate optical filter and a 10 mm cuvette were selected. Distilled water was used as a reference standard for comparing the results.Based on the type of temperature dependence observed in the turbidity values of the dispersions–relative to the dose of flocculant and the shape of the curves–the data can be conditionally divided into three groups (Figure 1). It was established that in the temperature range of 293–303 K, the sedimentation rate of dispersions increases steadily with increasing relative basicity of the flocculant, while in the range of 313–323 K, the dependence of sedimentation rate on relative basicity shows a maximum at a basicity of 67–73%. This, in turn, allows us to recommend these conditions for real–world wastewater treatment processes, as they significantly influence purification efficiency and are an integral part of the treatment mechanism.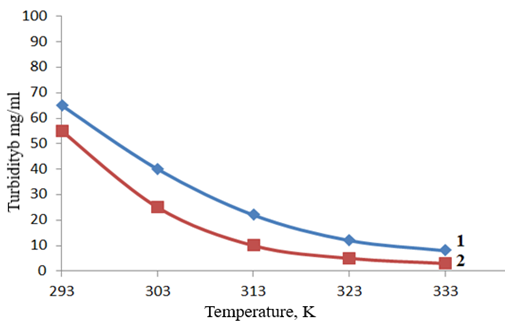 | Figure 1. Dependence of Turbidity of Na–CMC Flocculant Dispersions on Temperature at τ = 20 min. Na–CMC Concentration, mg/L: 1–0.25 mg/L; 2–0.20 mg/L |
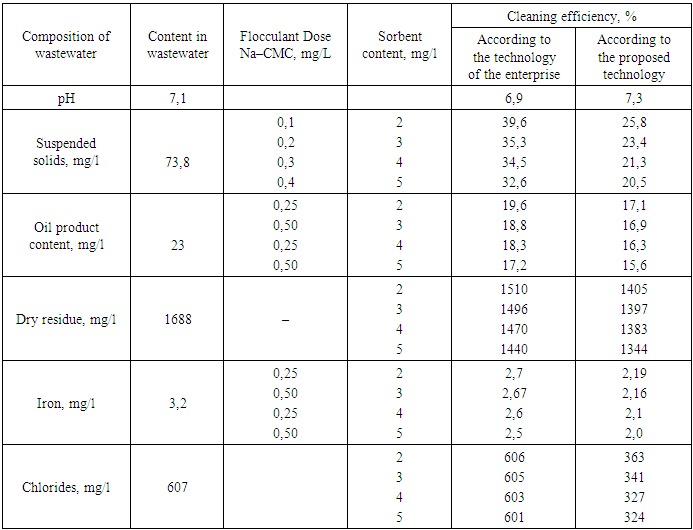
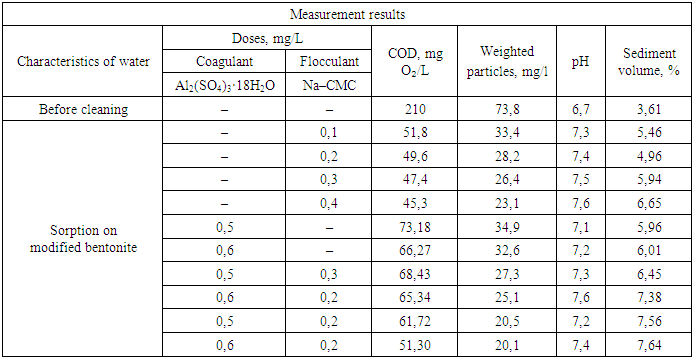
It should be noted that during adsorption, the molecules of the absorbed substance are concentrated on the surface of the modified bentonite under the influence of the surface force field [6-8].In turn, the surface force field is formed as a result of the presence of greater free energy in the boundary molecules of the solid phase, in contrast to the intraphase molecules. It is due to this that the boundary molecules attract molecules that are present in the contaminated wastewater, i.e. from the contacting phase. Taking into account this complex mechanism, which is accompanied by contact of the purified reagents with molecules or ions present in the composition of contaminated wastewater, depending on how the sorption phenomenon occurs on the surface of the sorbent, intermolecular interactions can be conditionally divided into three types: interaction between sorbent molecules and wastewater; between sorbent molecules and the extracted substance; between the molecules of the extracted substance and the wastewater. Accordingly, the difference in the energies of these three processes is the energy with which the substance extracted from the solution is retained on the surface of the sorbent. As is known, adsorption is a reversible process, and therefore, the sorbed substance can pass from the adsorbent back to the solution, i.e. desorption process occurs [9-13].Considering that the rate of sorption and desorption processes depends on the concentration of substances on the surface of the adsorbent and in the solution, we varied the doses of coagulant, flocculant, and slaked lime solution in order to reduce the chemical oxygen demand (COD) in wastewater, which reflects the degree of purification from the contaminants present.Our study on the efficiency of wastewater treatment from an oil refinery, depending on the doses of mineral coagulant and flocculant, as well as a 10% solution of slaked lime, allowed us to determine that the optimal doses for aluminum sulfate are 0.5 mg/L and for Na–CMC flocculant are 0.20 mg/L. The dose of the 10% slaked lime solution is 3 mL/L. Under these conditions, the treatment efficiency based on COD reaches 85–94%, the intensity of color removal is 93–96%, the reduction in petroleum product content is 72–74%, and the removal of iron ions is 66–68%.The research has shown that further increasing the doses of these mineral coagulant, flocculant, and lime solution has a minimal effect on improving the treatment efficiency. Therefore, the use of flocculants leads to an increase in the effectiveness of impurity removal. The results of experiments on studying the efficiency of wastewater treatment depending on the dose of Na–CMC flocculant are presented in Table 1.Table 1. The change in flocculant concentration during wastewater treatment with sorbents obtained on the basis of modified bentonite
 |
| |
|
The amount of coagulant in all experiments was 0.4 mg/l, and the amount of 10% slaked lime solution was 3 ml/l. salt content of the treated water. In order to assess the flocculating ability of Na–CMC in the wastewater treatment process, a series of experiments was conducted. It should be noted that, despite its higher cost, Na–CMC has certain advantages over mineral coagulants–it is more effective and can be used in significantly smaller doses. Additionally, it is non–corrosive, easily transportable, reduces the doses of mineral coagulants and, consequently, the volume of sludge, and does not increase the environmental impact.It should be noted that for maximum extraction of contaminants, the flocculation process should be carried out in the range of optimised pH values. We have experimentally determined that the greatest effect of purification of wastewater from oil refineries using Na–CMC as a flocculant is achieved in the range of pH values of the medium from 6.5 to 7.5.It is interesting that the required amounts of flocculant are expressed in terms of the smallest doses of polyacrylamide and carboxymethylcellulose, at which the intensity of wastewater coloration decreases by approximately 82–95%, depending on the initial composition of the water.We carried out further studies on the efficiency of wastewater treatment using the joint use of coagulants and flocculants, the results of which are presented in Table 2 and in Figure 2.Table 2. The changes in wastewater treatment performance depending on the dose of coagulants and flocculants when used together
 |
| |
|
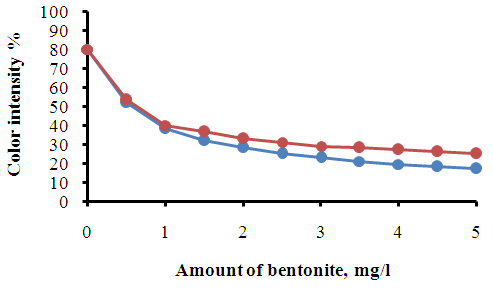 | Figure 2. The change in color intensity depending on the dose of modified sorbent in the presence of coagulant, flocculant and 10% solution of slaked lime. Coagulant dose–0.4 mg/l, flocculant dose–0.2 mg/l, and the dose of 10% solution of slaked lime–3 ml/l |
The obtained results show that the combined use of bentonite, flocculant and coagulants provides the most acceptable wastewater treatment efficiency compared to two– and three–component systems, with the purification efficiency for suspended solids reaching 96.0%.In our study of flocculant adsorption using the electrokinetic method, it was shown that the negative electrokinetic potential (EKP) of the particles of wastewater contaminants from the dyeing production decreases with an increase in the flocculant dose. Similar dependencies of the reduction in the negative value of EKP for dispersed contaminants on the flocculant dose were observed during the flocculation–based treatment of wastewater.The obtained results confirm the electrostatic nature of particle–floculant interactions for both low–molecular and high–molecular floculants, on the basis of which the mechanism of floculant adsorption is substantiated.It has been observed that the reason for the decrease in EKP of the particles upon the addition of low–molecular–weight polyelectrolytes such as PAA and CMC is the adsorption of macromolecules onto specific areas of the bentonite particles, which reduces the charge of these regions and, consequently, the overall charge of the particles. Such adsorption is possible because the size of the CMC polyelectrolyte macromolecules (0.075–0.1 µm) is significantly smaller than the size of the dispersed contaminant particles in the wastewater, which typically exceeds 5 µm.As a conclusion, high–molecular–weight flocculant CMC, whose macromolecule size reaches several tens of micrometers, adsorbs onto the surface of particles only partially through segments of the macromolecules. This also leads to a reduction in the electrokinetic potential.It should be noted that the extent of the reduction in the electrokinetic potential (EKP) of dispersed contaminants depends on the dose, type of flocculant, and the composition of the wastewater. A bentonite suspension, which can be considered a single–component system, when treated with flocculants, leads to a sharp reduction in the EKP of the bentonite particles (Table 3).Table 3. The electrokinetic potential of a sorbent suspension depending on the concentration of mineral coagulants and wastewater flocculants
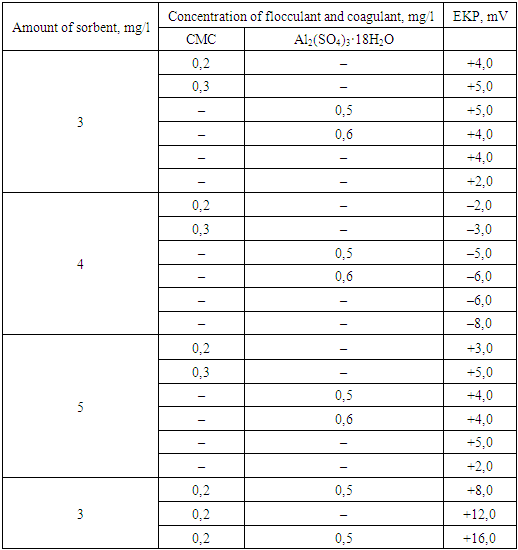 |
| |
|
As seen, the reduction in EKP of bentonite particles from (+5 mV) to (–8.0 mV) occurs at concentrations of Al₂(SO₄)₃·18H₂O ranging from 0.4–0.5 mg/L, which is significantly lower than the doses typically used for wastewater treatment (1.5–10.0 mg/L).Accordingly, with further increases in the flocculant dose, the electrokinetic potential (EKP) of the bentonite particles becomes positive and increases up to a certain limiting value, which corresponds to the adsorption saturation of the surface and depends solely on the type of flocculant. This limiting value of EKP was used in our work to assess the actual charge of the macromolecules of the flocculant in aqueous solutions, taking into account their conformational state.As seen, the optimal dose of flocculant corresponded to a certain negative value of EKP for the contaminant particles, which depended on the type of flocculant and the composition of the wastewater. That is, the greatest cleaning effect was observed when the particles were covered by a low–molecular–weight coagulant (Al₂(SO₄)₃·18H₂O), while they were only partially covered by the polymer. This can be explained by the process of flocculation of the dispersed phase particles with the adsorbed flocculant molecules (Table 4).Table 4. Electrokinetic characteristics of flocculant and dispersed wastewater contaminants during flocculation in the presence of a mineral coagulant
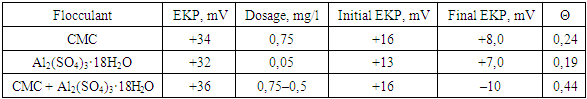 |
| |
|
Flocculant macromolecules can pass only in the presence of a free surface of contaminant particles [14]. The degree of filling of the surface with flocculant macromolecules (value θ) was proposed to be calculated using the formula: θ = (Initial electrokinetic potential (EKP)–Final electrokinetic potential (EKP)) / Flocking electrokinetic potential (EKP).Where initial electrokinetic potential (EKP initial) and final electrokinetic potential (EKP final) refer to the electrokinetic potential of the particles in the initial wastewater and after adding the given dose of flocculant; flocculant electrokinetic potential (EKP floc) is the charge of the flocculant (the electrokinetic potential value corresponding to the maximum adsorption of the flocculant on the bentonite particles).The characteristics of the flocculant and dispersed wastewater contaminants in the floculation process, obtained by us on the basis of electrokinetic measurements, are presented in Table 4 and serve for a comparative assessment of various flocculants and mineral coagulants, and for predicting the efficiency of their use.
3. Conclusions
Thus, a new coagulation–flocculation–adsorption method for wastewater treatment of complex composition has been developed, which includes stages of pH regulation, treatment with a composite reagent, sludge separation, adsorption, and filtration. The use of this method allows for the removal of dyes, surfactants, and complex organic and inorganic impurities with an efficiency of 97.0–98.7%.It has been shown that the use of composite reagents increases the sedimentation rate of coagulated and flocculated aggregates and the degree of purification compared to their aqueous analogs (previously dissolved or suspended reagents), simplifies the treatment process (reduces the number of technological stages for introducing reagents from 2–3 to 1), and also reduces the amount of coagulating agent and flocculant introduced by 1.2–1.5 times.Thus, new composite reagents have been developed and obtained, consisting of powdery coagulating agents such as aluminum sulfate and iron chloride, flocculants based on polyacrylamide and Na–CMC in a mass ratio of 1.0:1.0, sorbents at 2.0 g/l, and pH regulators. To achieve sequential action of reagents when introduced into the treated water, the coagulating agent particles should have a dispersion degree from 0.006 μm⁻¹ to 0.016 μm⁻¹, the flocculant–0.003 μm⁻¹, and the sorbent–0.02–0.05 μm⁻¹.
References
| [1] | Shikhaleva, E. P. (2012). Electrochemical coagulation method for wastewater treatment. Ecology of Production, (4), 62–69. |
| [2] | Grishin, B. M., & Salmin, S. M. (2012). Research on the reagent treatment of natural waters using aluminosilicate coagulants. In Current Problems of Engineering Sciences in Industry, Ecology, and Protection of Water Resources: Proceedings of the International Scientific and Practical Conference (pp. 116–119). Penza: PGUAS. |
| [3] | Domracheva, V. A., & Shiyravi, G. (2013). Adsorption extraction of heavy metal ions using carbon adsorbents in static conditions. Non-ferrous Metals, (1), 43–48. |
| [4] | Amonova, M. M., & Ravshanov, K. A. (2019). Influence of coagulant concentration on the degree of wastewater treatment. Development of Science and Technology. Scientific and Technical Journal, (2), 57–61. |
| [5] | Amonova, M. M., Ravshanov, K. A., & Amonov, M. R. (2019). Study of coagulant doses in wastewater treatment of textile industry. Universum: Chemistry and Biology, (6), 47–49. |
| [6] | Velyaev, Y. O., Mayorov, D. V., & Matveev, V. A. (2013). Study of the efficiency of using aluminosilicate coagulants based on nepheline. Water Supply and Sanitary Technology, (3, Part 1), 32–37. |
| [7] | Amonova, M. M., & Ravshanov, K. A. (2019). Study of the concentration of mineral sorbents in wastewater treatment of textile industry. Composite Materials, (3), 86–90. |
| [8] | Vikhrev, V. I., Simonov, A. D., & Shevchenko, V. S. (2013). Thermal disposal of wastewater sludge. Clean City, (1), 27–32. |
| [9] | Amonova, M. M., & Ravshanov, K. A. (2019). Polymeric composition for wastewater purification from various impurities in the textile industry. Journal of Chemistry and Chemical Technology, 62(10), 147–153. |
| [10] | Krylov, I. O., & Lugovskaya, I. G. (2011). Use of natural shungite sorbents in wastewater treatment systems. Resource-saving Technology. Express Information, (6), 10–32. |
| [11] | Selitsky, G. A., & Ermakov, D. V. (2011). Ways to improve the depth of treatment of acidic wastewater. Ecology of Production, (4), 70–78. |
| [12] | Amonova, M. M., & Ravshanov, K. A. (2019). Study of electrokinetic characteristics of flocculants and dispersed contaminants of wastewater from textile industry. Composite Materials, (1), 103–106. |
| [13] | Vigneswaran, S. (2009). Wastewater treatment technology (Vol. III). EOLSS Publications. |
| [14] | Arsentieva, N. A. (2013). Wastewater treatment: Bibliographic list of literature (Issue 4). National Library of the Chuvash Republic. |



 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML



