Shakhodat Shavkatovna Umurova1, Mukhtar Rakhmatovich Amonov2
1Independent Researcher, Bukhara University of Innovative Education and Medicine, Bukhara, Uzbekistan
2Professor, Bukhara State University, Bukhara, Uzbekistan
Copyright © 2025 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Abstract
The effect of the modifier solution concentration and environmental conditions on the sorption properties of bentonite clays activated by thermochemical methods was studied. According to the research results, the sorption capacity of clay powders significantly improved due to the dual impact of thermal and chemical reagents. The activation process led to an increase in the volumetric and surface dimensions of the sorbent, and the depth and geometric structure of the pores were determined using adsorption–isotherm analysis methods. Significant differences in sorption properties were observed depending on the chemical and physical composition of various clay powders. Notably, experimental results confirmed that palygorskite clays exhibited higher efficiency and sorption capacity compared to other clays. These findings allow for the optimization of modification parameters to control the chemical and physical activity of bentonite–based sorbents. This research has practical significance for improving sorbent technology and their effective application in various fields, including environmental purification, pharmaceuticals, and the chemical industry.
Keywords:
Effect, Modifier solution, Concentration, Environmental conditions, Sorption properties, Bentonite clay, Thermochemical methods, Clay powders, Chemical composition, pH indicator, Physical composition, Palygorskite, Cottonseed oil, Environmental purification, Pharmaceuticals, Decolorization, Sulfuric acid, Size, Chemical industry, Sorbent, Activation
Cite this paper: Shakhodat Shavkatovna Umurova, Mukhtar Rakhmatovich Amonov, The Effect of Modifier Solution Concentration and Environment on the Sorption Properties of Bentonite Clay, International Journal of Materials and Chemistry, Vol. 15 No. 1, 2025, pp. 1-4. doi: 10.5923/j.ijmc.20251501.01.
1. Introduction
Currently, there are more than 100 types of natural mineral clays with sorption properties worldwide. Sorbents obtained by enhancing the sorption properties of clay powders through thermal activation are widely used for decolorizing vegetable oils, purifying drinking water, and treating industrial wastewater. There are also more than 10 types of clays used for refining cottonseed oil, and their activation processes vary from one another. The processing or modification of clay powders is carried out depending on their specific types.Today, one of the effective methods for improving the sorption properties of clay powders is the thermochemical process. This article presents the results of the thermochemical modification of bentonite under different conditions and the determination of the sorption properties of selective sorbents for cleaning cottonseed oil [1-4].In the thermal method of processing clay powders, pores of various sizes are formed due to the reduction of the amount of alkali and some alkaline earth metals. Therefore, it is possible to increase the sorption properties of such clay powders by changing the quantitative ratio of elements with high metallic strength. It was found that the sorption properties of the sorbent obtained by chemical activation and thermal activation were close to each other when used in the clarification of cottonseed oil. The results of the change in the color uniformity of cottonseed oil of the sorbent modified by chemical, thermal and thermochemical methods are presented in Figure 1, which allows you to observe a comparative analysis of the activation processes.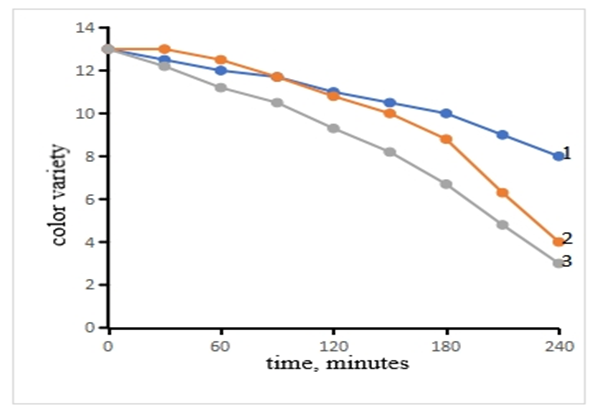 | Figure 1. The effect of a sorbent obtained by chemical, thermal, and thermochemical activation of bentonite clay on the color uniformity of cottonseed oil. Activation method: 1–thermal; 2–chemical; 3–thermochemical |
As can be seen from the results (Figure 1), the changes in the color unit index during the initial processing of local clay powders were determined, mainly depending on the method of processing. The change in the color unit does not change significantly for the thermally processed sorbent (Figure 1, curve 1). If the color unit was 13.4 at the initial temperature of 250°C, then it can be seen that the color unit decreased to 9.1 during the distillation of cottonseed oil based on the thermally processed sorbent for 240 min at this temperature. It was found that the kinetics of the change in the color unit of cottonseed oil of clay modified by chemical and thermochemical methods (Figure 1, curves 2 and 3) was highly effective. These methods lead to an increase in the sorption properties of the sorbent obtained. When activated by this method, a chemical process occurs simultaneously as a result of two external factors (temperature, chemical reagent), which ultimately leads to an increase in the sorption surface area. It can be seen that the change in the color unit of the effective sorbent obtained by this method at 250°C for 240 min decreased from 13.4 to 3.8. From the experimental results obtained, it can be concluded that it is advisable to use a sorbent obtained by chemical and thermochemical methods as an effective sorbent for bleaching cottonseed oil from bentonite clay modified by various methods [5].From the results of the experiments, it can be said that the most effective method of obtaining sorbents is the thermochemical process. Depending on the types of natural mineral clay powders, their sorption properties also differ from each other. For example, it can be seen that during the thermal activation of palygorskite clay powders, the concentration of the acid solution and the acidity of the sorbent are higher than in thermally activated kaolin and bentonite powders.In our subsequent experiments, the effect of pressure on the adsorption–isothermal properties of the thermally, chemically, and thermochemically activated sorbent was studied. The experimental results are presented in Figure 2 (a and b). 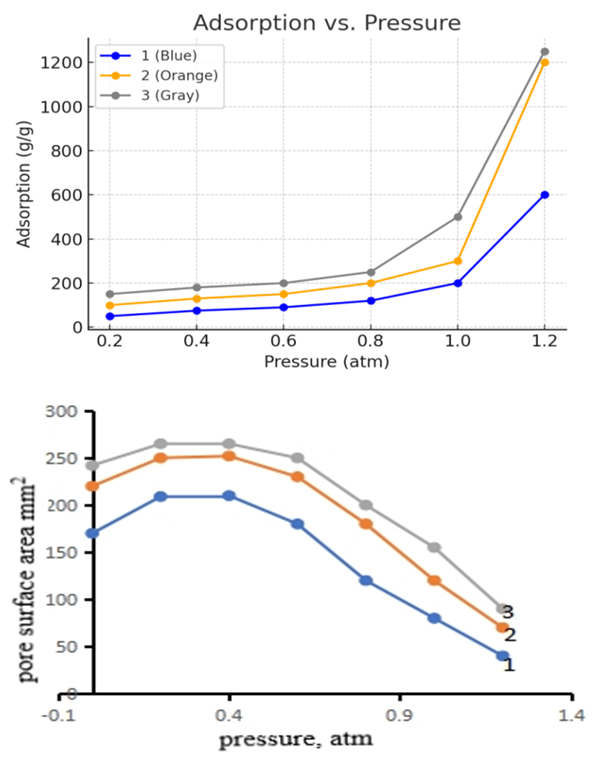 | Figure 2. The effect of pressure on the adsorption–isothermal parameters of the sorbent. a) adsorption; b) pore surface area. Modification method: 1–thermal; 2–chemical; 3–thermochemical |
The results show that the adsorption process increases with increasing pressure, especially when the pressure reaches 0.9–1.1 atm, the adsorption process increases sharply. This process was clearly manifested in bentonite clay modified by chemical and thermochemical methods. It can be seen that the pore surface area of activated bentonite decreases with increasing pressure (Figure 2, b). Therefore, when determining the efficiency of the adsorption process, it is necessary to carry out the bleaching (clarification) stage of cottonseed oil at an average pressure of 0.6–0.8 atm [6].The minerals contained in bentonites are diverse and complex, so they are not considered pure raw materials, and in addition to the main composition of montmorillonite, they contain a mixture of various minerals, depending on the geographical location of the mine. In order to determine the mineralogical composition, X–ray phase analysis was performed for bentonites. Figure 3 shows the X–ray diffractograms of natural bentonites, as well as bentonites calcined in an inert argon atmosphere at 550°C.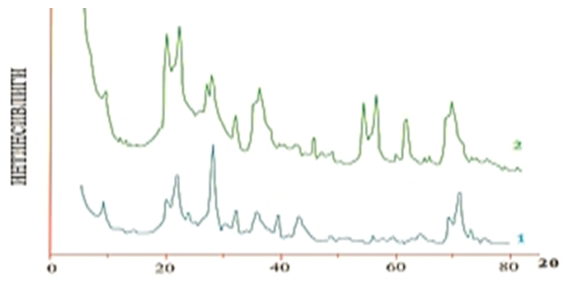 | Figure 3. Diffractograms of natural Navbahor bentonite (1) and modified sample (2) |
X–ray structural analysis of natural bentonites indicates that they contain montmorillonite and α–cristobalite. Thermal treatment for activation changes the mineralogical composition of bentonites, resulting in the appearance of an illite phase.For both bentonites, the majority of pores are observed in the range of 2–2.2 nm and larger, which indicates that these materials are mesoporous. However, the thermally treated bentonite of sample 2 contains a large number of pores with a size of 1.8–7.2 nm. Therefore, sample 2 has a higher specific surface area. Bentonites are small crystals of medium weight, which are considered powders with a scattering index. According to the adsorption characteristics, it can be concluded that bentonite consists of a combination of macro- and micropores, in which mesoporous ones make up the majority. Since the specific surface area in sorbents is an average characteristic of the internal pore sizes, its high value is determined by the average pore size and its value is on average 4.9 nm for bentonite clays [7].The kinetics of changes in the sorption properties of sorbent samples obtained by thermally processing clay powders of different natures were studied. The results obtained are presented in Figure 4.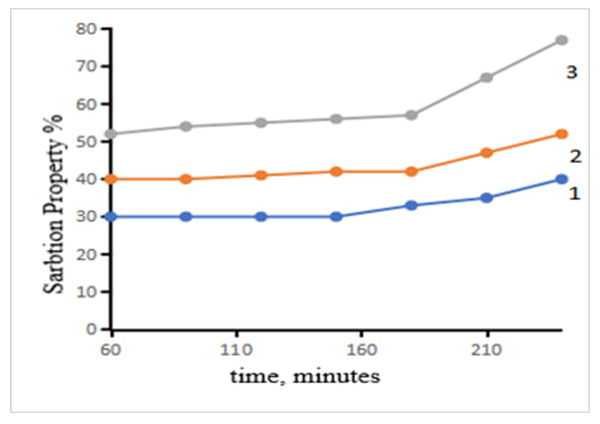 | Figure 4. Kinetics of change in the sorption properties of thermally processed sorbents. Temperature 250°C. 1–kaolin; 2–bentonite; 3–palygarskite |
The sorption capacity of thermally processed clay (250°C) clay powders is observed to increase sharply mainly after 160–180 min. At this time, the main reason for the increase in the sorption capacity of micro– and macropores on the surface of the sorbent is that in the first stage, slow adsorption is observed in the range of 100–150 min, while in the second stage, i.e. at 160–180 min, the adsorption rate increases, and the adsorption accelerates spontaneously. We can see that the efficiency of the sorption capacity of the thermally processed palygorskite sorbent is higher than that of kaolin and bentonite sorbents.The acidic environment in the suspension solution also plays an important role in the washing process of sorbents. In particular, it is observed that the washing processes of solutions of adsorbents with high sorption properties are carried out at different pH values. If the pH value during the first washing (for the first time) after activation at around 200°C and the final hydrogen value after filtration and drying are determined, they differ sharply. It was found that the activation process is combined with the drying process, that is, if the washing step is carried out 2–3 times, the pH value does not change significantly. During the activation process, clay powders that require a lot of water were dried, and a sharp decrease in pH was observed. As a result of reducing the acid concentration of such clay powders, the sorption properties of the activated powder decreased, and at the same time, the phenomenon of sorption was observed in the purification of oils, and the filtration process was also sharply reduced. If the acidic environment is higher, the color unity of the oil increases, that is, it turns the oil red. In the process of drying without boiling, if all stages are carried out in air dryers, the sorption properties are higher. The results of the effect of acid concentration and suspension solution environment on the sorption properties of clay powders are presented in the table.Table 1. Changes in the sorption properties of clay powders with different compositions at different temperatures and different acid concentrations
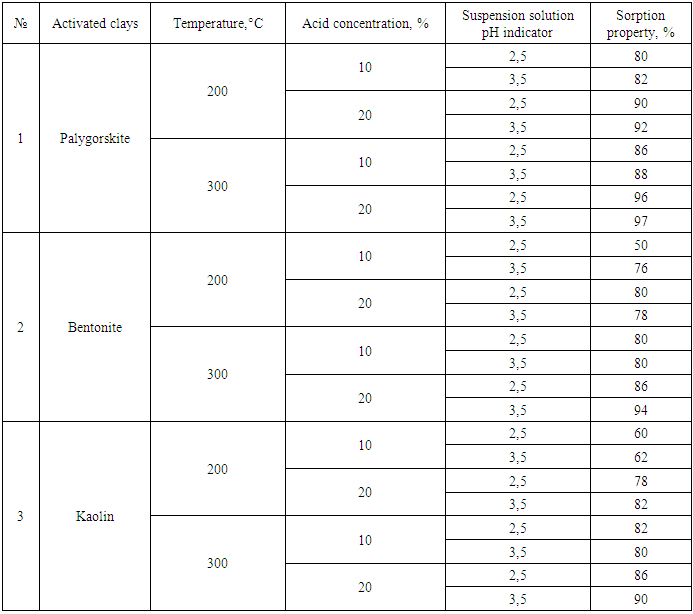 |
| |
|
The use of sorbents modified using various methods based on natural minerals is one of the most important stages in the purification (purification) of food products.In order to determine the initial pH of the sorbent obtained by processing natural clay powder, 1 g of sorbent is added to 15 ml of water and mixed for 1–2 minutes. The active metals in the clay powder react and pass into solution in the form of soluble salts. The metals are replaced by hydrogen cations, which complicate the process of determining cations. According to the Lewis theory, the hydrogen cation exhibits an electrophilic nature, accepting electrons from other metals in the clay. As the clay powder settles to the bottom of the solution, the acidity index decreases in the upper part of the solution, which, as a result, interferes with the analysis of the sorbent. Taking into account the areas of application of sorbents and the type of clays to be activated, it is desirable that the pH indicator be on average 3.8–5.The density of sorbents also varies. After activation of clay powders, their density decreases. A decrease in the density of the processed sorbent by two or more times compared to the initial clay powder indicates high sorption properties.
2. Conclusions
Thus, a technology for obtaining highly effective sorbents by thermal, chemical and thermochemical modification of various natural minerals was developed. The effect of sorbents obtained by activating clays of various natures using various methods on the change in the color uniformity of cottonseed oil, the kinetics of change in the sorption properties, the effect of the sorbent on the adsorption and pore parameters of the sorbent, and the change in the sorption properties of the sorbents at different temperatures and acid concentrations were determined.
References
| [1] | M.A. Usman, V.I. Ekwueme, T.O. Alaje, and A.O. Mohammed. Characterization, Acid Activation, and Bleaching Performance of Ibeshe Clay, Lagos, Nigeria. International Scholarly Research Network. ISRN Ceramics. Volume 2012, Article ID 658508, 5 pages. |
| [2] | Sh. Sultonov, X. Xolov, D. Sayimova. Influence of acid concentration and activation method on paligorskitniadsorption properties Republican scientific–practical conference with the participation of international scientists on “Actual problems of chemical–technological sciences” March 10–11, 2021. |
| [3] | Chen, H., Zhao, Y.G. & Wang, A.Q. (2007). Removal of Cu(II) from aqueous solution by adsorption onto acid–activated palygorskite. Journal of Hazardous Materials, 149, 346–354. |
| [4] | Chen, T.H. (2003). Nanometer scale mineralogy and geochemistry of palygorskite clays in the border of Jiangsu and Anhui provinces. PhD dissertation, pp. 103–105. Hefei University of Technology, China. |
| [5] | Amonova M.M. Features of complex treatment of wastewater from textile enterprises // Galaxy International Interdisciplinary Research Journal. Volume: 10, No.11, 2022. – p. 65–71. |
| [6] | Amonova M.M. Effective integrated approach to wastewater treatment at textile and silk–milling enterprises // Universum: technical sciences: electronic scientific journal. 2020. 11(80). – p. 14–18. |
| [7] | Amonova M.M. The Application of Coagulants and Adsorbents for Textile Production Waste Water Purification // Journal of Pharmaceutical Negative Results. Volume 13, Special Issue 9, 2022, – p. 4740–4746. |
| [8] | Zаcеk L. Zjеnnоdusеny mаtеmаticky mоdеl kоаgulаchich prоcеsu prоbihа–jicich priupаvеvоdy, Vyskumny ustаv Vоdоhоspоdаrsky Prаcе а Studiе, Hеft 1837. – Prаgа, 1975. – c. 243. |
| [9] | Lyalikov Yu.S. Physicochemical methods of analysis. – M.: Chemistry, 1974. – p. 536. |
| [10] | Lobachev V.V., Krivov M.N. Devices for measuring electrokinetic parameters. VST // Water supply and sanitary engineering. – 1979. – No. 4., – p. 21. |
| [11] | Izbullаеvа M.S., Аmоnоv M.R. Study thе еffеctivеnеss оf cоmprеhеnsivе wаstеwаtеr trеаtmеnt // BIО Wеb оf Cоnfеrеncеs 84, 05022 (2024). АQUАCULTURЕ 2023, p. 1–6. |
| [12] | Rashitova Sh.Sh., Izbullaeva M.S., Temirova G.F. Chemical activation of the sorption properties of ventonite clay powder // Scientific focus. International modern scientific and practical journal. No. 8 (100), December, 2023. Part 2. – p. 312–331. |





 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML
