-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
International Journal of Materials and Chemistry
p-ISSN: 2166-5346 e-ISSN: 2166-5354
2024; 14(6): 112-119
doi:10.5923/j.ijmc.20241406.04
Received: Dec. 15, 2024; Accepted: Dec. 30, 2024; Published: Dec. 31, 2024

Catalytic Synthesis of High-Molecular Hydrocarbons from Synthesis Gas
Normurot Fayzullaev 1, Asliddin Mamatov 2
1Doctor of Technical Sciences, Professor, Department of Polymer Chemistry and Chemical Technology, Samarkand State University, Samarkand, Republic of Uzbekistan
2Doctoral Student of Samarkand State University, Samarkand, Uzbekistan
Correspondence to: Normurot Fayzullaev , Doctor of Technical Sciences, Professor, Department of Polymer Chemistry and Chemical Technology, Samarkand State University, Samarkand, Republic of Uzbekistan.
| Email: |  |
Copyright © 2024 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

This study investigates the catalytic synthesis of high molecular weight synthetic hydrocarbons, ranging from pentane to nonadecane, using a flow reactor operating in differential mode. The textural properties of catalysts designed for synthesizing these hydrocarbons from synthesis gas were characterized through nitrogen adsorption and desorption isotherms, employing a Sorbtometer device. Key parameters analyzed include total relative surface area, average particle size, mesopore volume, and pore size distribution, with the BJH method used to determine the micropore and mesopore volumes. The surface morphology of the catalysts was further examined through scanning electron microscopy and light scattering microscopy. The primary objective of this research is to elucidate the kinetic principles governing the synthesis of high molecular weight hydrocarbons from synthesis gas.
Keywords: Syngas, High molecular weight hydrocarbons, Catalyst, Kinetic principles, Textural properties
Cite this paper: Normurot Fayzullaev , Asliddin Mamatov , Catalytic Synthesis of High-Molecular Hydrocarbons from Synthesis Gas, International Journal of Materials and Chemistry, Vol. 14 No. 6, 2024, pp. 112-119. doi: 10.5923/j.ijmc.20241406.04.
Article Outline
1. Introduction
- In order to improve the lifestyle of people around the world, the sudden increase in energy demand in the development of industry and transportation sectors causes some problems [1]. Developing alternative and environmentally friendly ways to produce liquid fuels is a difficult and demanding task. Fischer-Tropsch (FT) synthesis and similar technologies such as gas-to-liquids (GTL), coal-to-liquids (CTL) and biomass-to-liquids (BTL) are alternative methods to replace oil. They can make a positive contribution to the world's energy security and supply. Although the FT process was discovered about a century ago, it is an attractive and alternative source of environmentally friendly liquid hydrocarbon fuels with sulfur-free and aromatic compounds. Fischer-Tropsch synthesis is a catalytic reaction in which syngas (a mixture of CO and H2) is converted into liquid hydrocarbon fuel [2,3]. FT synthesis has received much attention worldwide in both industrial and scientific fields, with the catalyst being the heart of the process. Catalyst composition and operating parameters such as temperature, pressure, etc. play an important role in FT synthesis, catalyst activity, and product distribution. Thus, it is essential to develop FT catalysts with high activity, selectivity, and stability. Among the intermediate metals, ruthenium, nickel, cobalt, and iron are the most commonly used metals for FT catalyst. Cobalt is an active metal for the synthesis of higher chain hydrocarbons due to its high activity and selectivity to paraffin, low water-gas exchange (WGS) activity, low CO2-selectivity and less oxygen generation [4-6]. Many researchers have studied the effect of different oxide supports on the activity/selectivity of CO catalysts [7,8]. Al2O3, SiO2, and TiO2 are the most commonly used materials for Co catalysts, in Co/Al2O3 catalyst, due to the limited reducibility of Co and strong interaction between Co [9-11]. Promoters such as Pd, Pt, Re, and Ru are added to Co catalysts, which promote better Co reduction with strong interactions with nonmetals other than Al2O3 [12,13]. Many researchers believe that the Pd promoter improves the adsorption sites of the Co catalyst and thereby increases the rate of hydrogenation in FT synthesis [14-16].In general, the FTS process involves two main reactions and several side reactions. The synthesis of long-chain paraffin and olefins consists of two main reactions [17-21]:
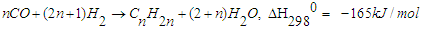 | (1) |
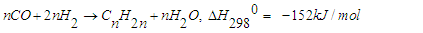 | (2) |
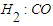 when the ratio is high or when hydrogenation is strong, catalysts such as cobalt and nickel are used, and reaction (1) becomes dominant. Unlike; on the other hand, when the H2:CO ratio is low or hydrogenation catalysts such as iron are used, reaction (2) dominates. Depending on the operating conditions and the nature of the catalysts, in addition to the two main reactions, the methanization reaction (3), the reaction of the formation of oxygen compounds of hydrocarbons (4) and the reaction of CO2 formation (5) can occur:
when the ratio is high or when hydrogenation is strong, catalysts such as cobalt and nickel are used, and reaction (1) becomes dominant. Unlike; on the other hand, when the H2:CO ratio is low or hydrogenation catalysts such as iron are used, reaction (2) dominates. Depending on the operating conditions and the nature of the catalysts, in addition to the two main reactions, the methanization reaction (3), the reaction of the formation of oxygen compounds of hydrocarbons (4) and the reaction of CO2 formation (5) can occur: | (3) |
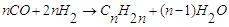 | (4) |
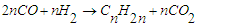 | (5) |
2. Experimental Part
- Catalytic properties of catalysts in the synthesis of hydrocarbons from CO and H2 were investigated in a flow isothermal reactor filled with 30 cm3 of quartz (catalyst layer 10 cm3) under the following optimal conditions: temperature range 280-300°C, 0.5 MPa and volumetric gas flow rate (GHT) 1000 h-1. Balanced experiments were carried out for at least 150 hours, the composition of the incoming and outgoing gas was analyzed, and the amount of hydrocarbons and water of reaction obtained was recorded.The activity of hydrocarbon synthesis catalysts was evaluated according to the following indicators: CO conversion, selectivity for hydrocarbons and productivity. The calculation error did not exceed 2.5%. CO conversion was calculated according to the following formula:Catalytic properties of catalysts in the synthesis of hydrocarbons from CO and H2 were investigated in a flow isothermal reactor filled with 30 cm3 of quartz (catalyst layer 10 cm3) under the following optimal conditions: temperature range 280-300°C, 0.5 MPa and volumetric gas flow rate (GHT) 1000 h-1. Balanced experiments were carried out for at least 150 hours, the composition of the incoming and outgoing gas was analyzed, and the amount of hydrocarbons and water of reaction obtained was recorded.The activity of hydrocarbon synthesis catalysts was evaluated according to the following indicators: CO conversion, selectivity for hydrocarbons and productivity. The calculation error did not exceed 2.5%. CO conversion was calculated according to the following formula:
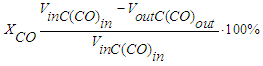 | (6) |
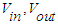 - gas consumption at the entrance to the reactor and the exit from the reactor, dm3/hour;
- gas consumption at the entrance to the reactor and the exit from the reactor, dm3/hour;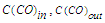 - concentration of CO at the inlet and outlet of the reactor, unit percentage.Methane selectivity was calculated according to the following formula:
- concentration of CO at the inlet and outlet of the reactor, unit percentage.Methane selectivity was calculated according to the following formula: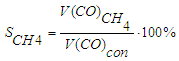 | (7) |
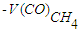 - the volume of CO used to produce methane, dm3;
- the volume of CO used to produce methane, dm3;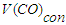 - volume of CO converted to methane, dm3.The selectivity for C5+ hydrocarbons was calculated using the formula:
- volume of CO converted to methane, dm3.The selectivity for C5+ hydrocarbons was calculated using the formula: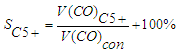 | (8) |
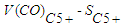 the volume of CO used to form hydrocarbons dm3.
the volume of CO used to form hydrocarbons dm3. - For hydrocarbons, productivity was developed according to the following formula:
- For hydrocarbons, productivity was developed according to the following formula: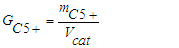 | (9) |
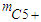 - mass of hydrocarbons, kg;
- mass of hydrocarbons, kg;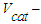 catalyst volume, m3;
catalyst volume, m3;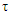 - time, hour.
- time, hour.2.1. Determination of the Composition of Synthesis Products
- The composition of the gaseous synthesis products was analyzed by gas adsorption chromatography and two columns with an active Hysep R phase and NaX molecular sieves in a Crystall 5000 (Chromatek, Russia) chromatograph equipped with a thermal conductivity detector. The assay mode is temperature programmable with a heating rate of 8°C/min.The composition of C5+ hydrocarbons was determined by the capillary gas-liquid chroma-mass spectrometric method on a gas chromatograph (Agilent, USA) equipped with an MSD 5975C mass-selective detector.
2.2. Texture Characteristics of Catalysts
- Textural characteristics of catalysts designed for the production of high molecular synthetic, pentane to nonadecane hydrocarbons from synthesis gas were determined on the basis of absorption and desorption isotherms of nitrogen in the Sorbtometer device, based on total specific surface area, average particle size, mesopore size, and pore size distribution. The volume of micropores and mesopores was determined by the BJH method. The surface-to-surface ratio was calculated by the Brunauer-Emmett-Taylor (BET method):
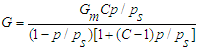 | (10) |
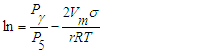 | (11) |
 | Figure 1. Absorption and desorption isotherms of benzene vapors |
2.3. Surface Morphology of Catalysts Designed for the Production of High Molecular Weight Synthetic Hydrocarbons from Pentane to Nonadecane from Syngas
- The morphology of the surface of the catalysts used in synthesis gas, i.e. in synthesis gas, for obtaining high molecular synthetic hydrocarbons from synthesis gas, from pentane to nonadecane, in oxidized and reduced forms, by scanning electron and radiation microscopy method was studied.According to light microscopy data, changes in the composition of catalysts designed for the synthesis of high-molecular-weight synthetic hydrocarbons from pentane to nonadecane do not affect the distribution of cobalt particles (Fig. 2), the size of the initial gas and high-molecular synthetic hydrocarbons from hydrogen, from pentane to nonadecane Co- Fe-B-Zr/HSZ Synthesis-gas high molecular synthetic, from pentane. The average value of cobalt particles in the catalyst designed for the production of hydrocarbons up to nonadecane corresponds to the size of 8.3 nm, which is 3-13 nm.
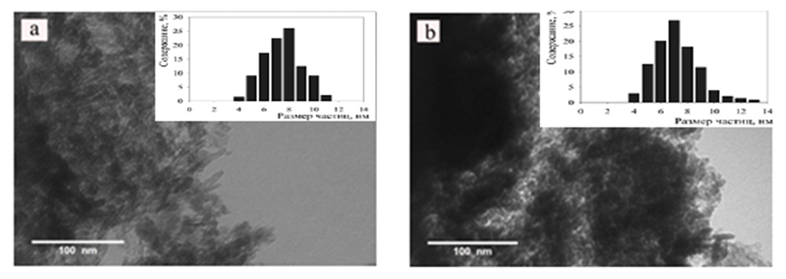 | Figure 2. PEM photomicrographs of regenerated Co-Fe-B-Zr/HSZ catalysts with different compositions %: a – 3%B-2%Zr; b – 5%B-0.5%Zr |
3. Results and Discussion
3.1. The Effect of the Method of Preparing a Catalyst for the Production of High-Molecular Hydrocarbons from Synthesis Gas with High Catalytic Activity on the Reaction Yield in the Synthesis of High-Molecular Liquid Synthetic Hydrocarbons
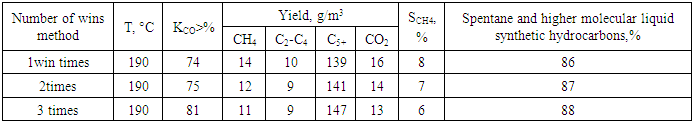
- Preparation of catalysts designed for obtaining high molecular weight hydrocarbons from synthesis gas with high catalytic activity selected for obtaining high molecular weight liquid synthetic hydrocarbons from syngas can be carried out in one or more steps. In the latter case, the active ingredients were introduced to the surface of the carrier by successive absorption with aqueous solutions of the respective salts. In our experiments on the effect of the catalyst activity enhancer for obtaining high molecular weight hydrocarbons from synthesis gas with high catalytic activity on the activity of the catalyst intended for obtaining high molecular weight hydrocarbons from synthesis gas with high catalytic activity selected for obtaining high molecular weight liquid synthetic hydrocarbons from synthesis gas the activity of a catalyst designed to obtain high molecular weight hydrocarbons from syngas with high catalytic activity zirconium by three consecutive absorptions of samples prepared with the introduction of a raising agent. Such a methodology does not create difficulties for laboratory tests, for which small amounts of the catalyst intended for obtaining high molecular weight hydrocarbons from synthesis gas with high catalytic activity are required.Tests were conducted under the following optimal conditions: pressure 0.1 MPa, volume velocity 100 h-1. The results were compared with the sample values prepared in 3 dilutions.As can be seen in Fig. 3, the sample prepared in 3-fold digestion was more active in the entire temperature range - the greenhouse gas conversion in it was 5-10% higher than that of the other two samples. In this case, the samples prepared 1 and 2 times were almost indistinguishable from each other.
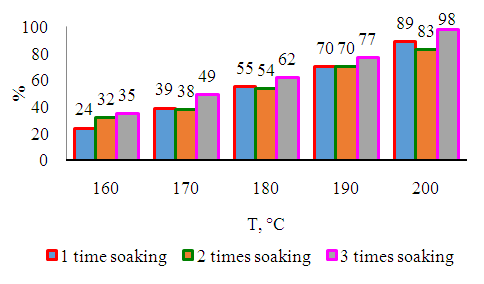 | Figure 3. Dependence of heat gas conversion on synthesis temperature |
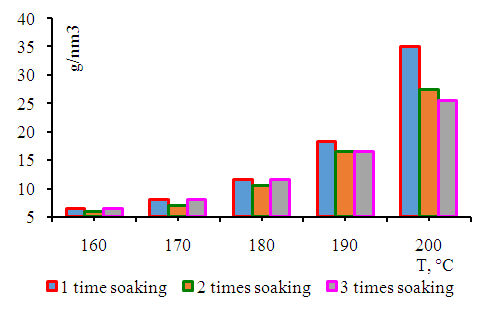 | Figure 4. Dependence of methane yield on synthesis temperature |
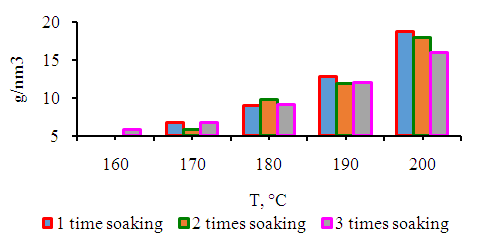 | Figure 5. Dependence of the yield of ethane, propane, butane and ethylene, propene, butenes on synthesis temperature |
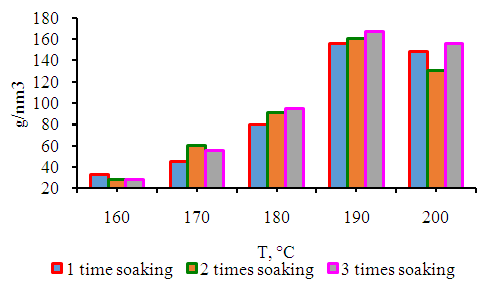 | Figure 6. Dependence of yield of liquid synthetic hydrocarbons on synthesis temperature |
|
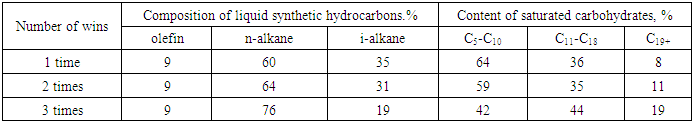
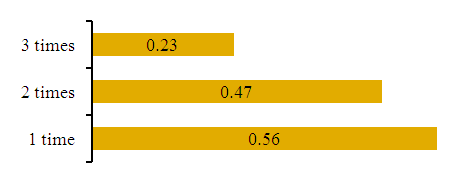 | Figure 7. Effect of amount of ingestion on iso/n-unsaturated carbohydrate ratio |
3.2. Effect of B-Zr Content in the Catalyst Selected for the Production of High Molecular Weight Liquid Synthetic Hydrocarbons from 20%Co-20%Fe-5%B-1.5%Zr/HSZ Synthesis Gas on Hydrocarbon Synthesis
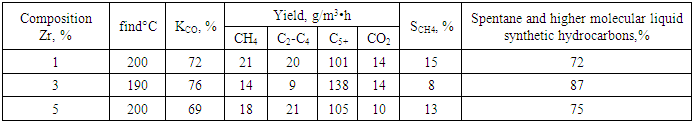
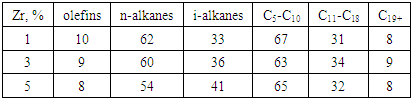
- The material additive composition of the catalyst designed to obtain high molecular hydrocarbons from synthesis gas with high catalytic activity, which increases the activity, affects the synthesis of high molecular synthetic hydrocarbons from carbon dioxide and hydrogen, from pentane to nonadecane. The sequence of unloading the components also has a significant impact on the process. 5%B-1%Zr/HSZ for the highest activity of the catalyst designed to obtain high molecular weight synthetic hydrocarbons from synthesis gas with high catalytic activity with the presence of a substance that increases the activity of the catalyst intended for The amount of the substance increasing the activity of the catalyst intended for obtaining high molecular hydrocarbons from synthesis gas with high catalytic activity affects the activity of the catalyst intended for obtaining high molecular hydrocarbons from synthesis gas with high catalytic activity selected for obtaining high molecular liquid synthetic hydrocarbons from synthesis gas. Thus, the sample containing 1% Zr and 5% B was more active. It achieved ~20% higher conversion compared to two other syngas high molecular weight liquid synthetic hydrocarbon catalysts with high catalytic activity selected for synthesis gas high molecular weight hydrocarbons.Tables 3, and 4 show performance indicators of catalysts designed for obtaining high molecular weight hydrocarbons from synthesis gas with high catalytic activity selected for obtaining high molecular weight liquid synthetic hydrocarbons from synthesis gas. Thus, the selectivity for C5+ in the optimum Zr (3%) content was 85% against 74-74% in the 0.5% content of catalyst 2 and 0.5%, which is intended for the production of high molecular hydrocarbons from syngas with high catalytic activity (3- table).
|
|
3.3. Effect of Total Pressure on the Synthesis of High Molecular Weight Synthetic, Pentane to Non-Decane Hydrocarbons from Carbon Dioxide and Hydrogen
- 20%Co-20%Fe-5%B-1.5%Zr/HSZ is selected by us for obtaining high molecular liquid synthetic hydrocarbons from synthesis gas with high catalytic activity in the catalyst designed for obtaining high molecular hydrocarbons from synthesis gas and hydrogen effect on the synthesis of high molecular weight synthetic hydrocarbons from pentane to nonadecane was studied. Experiments on the synthesis of high molecular liquid synthetic hydrocarbons from carbon dioxide and hydrogen were carried out at a pressure of 0.5-0.6 MPa and a volume velocity of 2000-2500 h-1, raising the temperature from 60 to 190°C. When this temperature was reached, the pressure was increased to 2.0 MPa in 0.5 MPa steps.An increase in the activity of the catalyst selected for obtaining high molecular weight synthetic liquid hydrocarbons from syngas with high catalytic activity was observed when the pressure was increased - the gas conversion increased from 11% at 0.5 MPa to 19% at 2.0 MPa. The increase in activity was observed with the production of liquid synthetic hydrocarbons. As the pressure increased from 0.5 to 2.0 MPa, the yield increased from 28 to 41 g/m3. Accordingly, the productivity of liquid synthetic hydrocarbons also increased from 55 to 89 kg/m3 cat.h (Figures 8, 9).When the pressure increased from 0.5 to 2 MPa, the selectivity to higher molecular weight liquid synthetic hydrocarbons than pentane increased from 93 to 96% (Fig. 8).
|
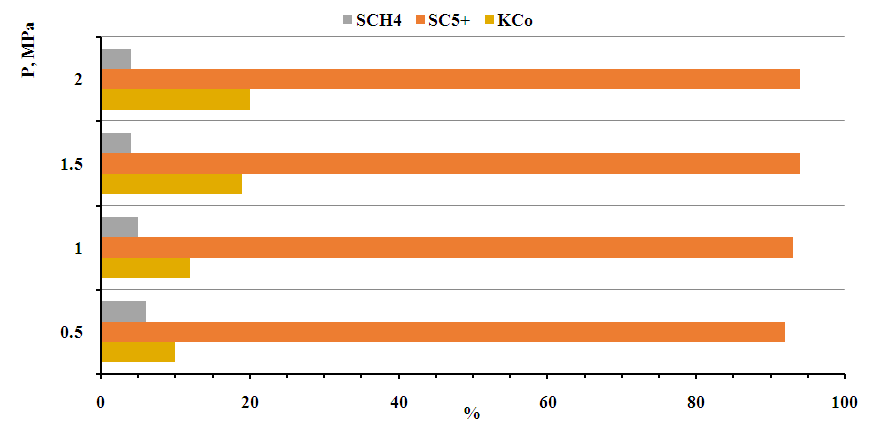 | Figure 8. Effect of pressure on the activity and selectivity of a selected catalyst for the production of high molecular weight liquid synthetic hydrocarbons from 20%Co-20%Fe-5%B-1.5%Zr/HSZ synthesis gas. T=190°C, space velocity 2000 h-1 |
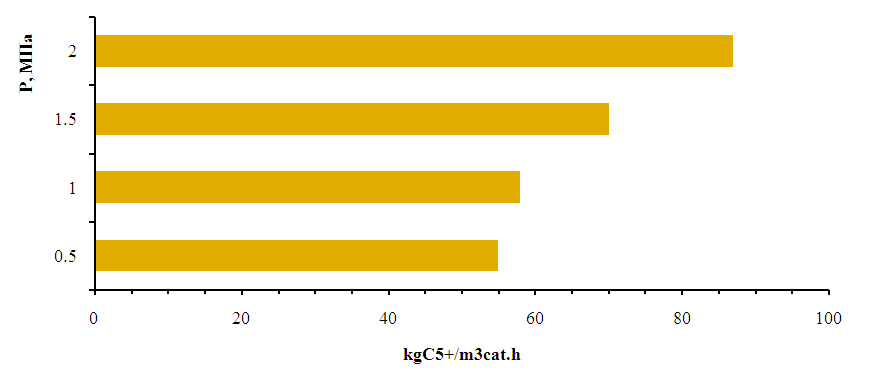 | Figure 9. Effect of pressure on the performance of a selected catalyst for the production of high molecular weight liquid synthetic hydrocarbons from 20%Co-20%Fe-5%B-1.5%Zr/HSZ synthesis gas. T=190°C, space velocity 2000 h-1 |
4. Conclusions
- The obtained data are placed into certain laws of the effect of pressure on the activity and selectivity of catalysts designed for obtaining high molecular hydrocarbons from synthesis gas with high catalytic activity selected for the synthesis of high molecular liquid synthetic hydrocarbons from synthesis gas.Thus, the catalytic synthesis of high molecular weight synthetic hydrocarbons from carbon dioxide and hydrogen, from pentane to nonadecane. The process was carried out in a flow reactor operating in differential mode. Textural characteristics of catalysts designed for obtaining high-molecular-weight synthetic hydrocarbons from pentane to nonadecane from syngas were determined. The volume of micropores and mesopores was determined by the BJH method. The morphology of the surface of the catalysts designed for the production of high molecular weight synthetic hydrocarbons from pentane to nonadecane from syngas was studied by scanning electron microscopy and light microscopy.
References
| [1] | Lešnik, Luka, Breda Kegl, Eloísa Torres-Jiménez, and Fernando Cruz-Peragón. "Why we should invest further in the development of internal combustion engines for road applications." Oil & Gas Science and Technology–Revue d’IFP Energies nouvelles 75 (2020): 56. |
| [2] | Maity, Sudip, Olusola O. James, Biswajit Chowdhury, and Aline Auroux. "Effect of copper on calcium-modified alumina-supported cobalt catalysts towards Fischer–Tropsch synthesis." Current Science (2014): 1538-1547. |
| [3] | Abbasi, Saeid, Mohsen Abbasi, Firouz Tabkhi, and Benyamin Akhlaghi. "Syngas production plus reducing carbon dioxide emission using dry reforming of methane: utilizing low-cost Ni-based catalysts." Oil & Gas Science and Technology–Revue d’IFP Energies nouvelles 75 (2020): 22. |
| [4] | Wang, Da, Zhong Wang, Guangci Li, Xuebing Li, and Bo Hou. "SiO2-Modified Al2O3@ Al-Supported Cobalt for Fischer–Tropsch Synthesis: Improved Catalytic Performance and Intensified Heat Transfer." Industrial & Engineering Chemistry Research 57, no. 38 (2018): 12756-12765. |
| [5] | Yao, Man, Nan Yao, Yan Shao, Qian Han, Chengcheng Ma, Changkun Yuan, Chengen Li, and Xiaonian Li. "New insight into the activity of ZSM-5 supported Co and CoRu bifunctional Fischer–Tropsch synthesis catalyst." Chemical Engineering Journal 239 (2014): 408-415. |
| [6] | Tursunov, Obid, Leonid Kustov, and Aleksandr Kustov. "A brief review of carbon dioxide hydrogenation to methanol over copper and iron-based catalysts." Oil & Gas Sciences and Technology–Revue d’IFP Energies nouvelles 72, no. 5 (2017): 30. |
| [7] | Jacobs, Gary, Tapan K. Das, Yongqing Zhang, Jinlin Li, Guillaume Racoillet, and Burtron H. Davis. "Fischer–Tropsch synthesis: support, loading, and promoter effects on the reducibility of cobalt catalysts." Applied Catalysis A: General 233, no. 1-2 (2002): 263-281. |
| [8] | Jacobs, Gary, Yaying Ji, Burtron H. Davis, Donald Cronauer, A. Jeremy Kropf, and Christopher L. Marshall. "Fischer–Tropsch synthesis: Temperature programmed EXAFS/XANES investigation of the influence of support type, cobalt loading, and noble metal promoter addition to the reduction behaviour of cobalt oxide particles." Applied Catalysis A: General 333, no. 2 (2007): 177-191. |
| [9] | Fayzullayev, N. I.; Umirzakov, R. R. To obtain acetone by spontaneous hydration of acetylene. ACS National Meeting Book of Abstracts. 2005. Vol. 229, pp. U598-U598. Web of Science Core Collection https://www.webofscience.com/wos/woscc/full-record/WOS:000235066602537. |
| [10] | Muradov, K. M., Fayzullayev, N. I., & Zohidov, K. A. Investigation of influence of various factors to oxidative condensation of methane in C2-hydrocarbons. In Abstracts of Papers of the American Chemical Society. 2003. Vol. 226, pp. U258-U259. 1155 16TH ST, NW, Washington, DC 20036 USA: Amer Chemical Soc. https://www.webofscience.com/wos/woscc/full-record/WOS:000187062501250. |
| [11] | Fayzullaev, N. Gas chromatographic study of catalytic steam-phase hydration of acetylene. In Abstracts of Papers of the American Chemical Society. 2003. Vol. 225, pp. U112-U112. 1155 16TH ST, NW, Washington, DC 20036 USA: Amer Chemical Soc. https://www.webofscience.com/wos/woscc/full-record/WOS:000187917800439. |
| [12] | Fayzullayev, N. I. Optimization process of synthesis of acetone from acetylene. In Abstracts of Papers of the American Chemical Society. 2002. Vol. 224, pp. U279-U279. 1155 16TH ST, NW, Washington, DC 20036 USA: Amer Chemical Soc. https://www.webofscience.com/wos/woscc/full-record/WOS:000177422301495. |
| [13] | Akmalaiuly K.; Fayzullayev N. Heterogeneo-catalytic synthesis of vinyl chloride and chloroprene from acetylene. Ser. Chem. Technol. 2020, 5 (443), 6–13. https://doi.org/10.32014/2020.2518-1491.74. |
| [14] | Fayzullaev, N. I.; Javkharov, J. Kinetical Laws of Catalytic Oxychlorination of Methanes. AIP Conf. Proc. 2023, 2789 (1). https://doi.org/10.1063/5.0149606/2899913. |
| [15] | Fayzullayev, N.; Akmalaiuly, K.; Karjavov, A. Catalytic synthesis of a line by acetylene hydration. Ser. Chem. Technol. 2020, 2 (440), 23–30. https://doi.org/10.32014/2020.2518-1491.19. |
| [16] | Fayzullayev, N. I.; Umirzakov, R. R.; Pardaeva, S. B. Study of acetylating reaction of acetylene by gas chromatographic method. In ACS National Meeting Book of Abstracts (PETR-66). 2005. Vol. 229, pp.U597-U597. Web of Science Core Collection https://www.webofscience.com/wos/woscc/full-record/WOS:000235066602532. |
| [17] | Fayzullayev, N. I. Optimization process of gas chromatographic separation products of catalytic synthesis of vinyl chloride. In Abstracts of Papers of the American Chemical Society. 2002. Vol. 224, pp. U125-U125. 1155 16TH ST, NW, Washington, DC 20036 USA: Amer Chemical Soc. https://www.webofscience.com/wos/woscc/full-record/WOS:000177422200508. |
| [18] | Fayzullaev, O. O., Fayzullayev, N. I., Muradov, K. M. Synthesis of ethylene from methane on piled catalysts. In Abstracts of Papers of the American Chemical Society. 2002; Vol. 223, pp. U25-U25. 1155 16TH ST, NW, Washington, DC 20036 USA: Amer Chemical Soc. https://www.webofscience.com/wos/woscc/full-record/WOS:000176296700080. |
| [19] | Muradov, K. M., Fayzullayev, N. I., Fayzullaev, O. O. Catalytic synthesis of nitriles from alcohols and ammonia. In Abstracts of Papers of the American Chemical Society. 2002; Vol. 223, pp. U26-U26. 1155 16TH ST, NW, Washington, DC 20036 USA: Amer Chemical Soc. https://www.webofscience.com/wos/woscc/full-record/WOS:000176296700088. |
| [20] | Fayzullaev, N.; Tursunova, N.; Xolmirzayeva, H. Kinetics and Mechanisms of Methane Oxycondensation Reaction. In AIP Conf. Proc. 2023, 2789 (1). https://doi.org/10.1063/5.0145622. |
| [21] | Sarimsakova, N.; Fayzullaev, N.; Mallaboev, N. Study of Adsorbation Properties of Sorbent Received on the Basis of Acidic Modication of Clinoptylotitis from Karmana Deposit. In AIP Conference Proceedings; American Institute of Physics Inc., 2023; Vol. 2789. https://doi.org/10.1063/5.0145548. |
| [22] | Xolmirzayeva, H. N.; Fayzullayev, N. I. Obtaining Nanocarbon from Local Raw Materials and Studying Its Textural and Sorption Properties. Int. J. Eng. Trends Technol. 2022, 70 (2), 163–171. https://doi.org/10.14445/22315381/IJETT-V70I2P219. |
| [23] | Fayzullayev, N. I., Muradov, K. M., Fayzullaev, O. O., Umirzakov, R. R., Pardaeva, S. B. About mechanism of acetone formation from acetylene on polyfunctional catalyst. In Abstracts of Papers of the American Chemical Society. 2002. Vol. 223, pp. U22-U22. 1155 16TH ST, NW, Washington, DC 20036 USA: Amer Chemical Soc. https://www.webofscience.com/wos/woscc/full-record/WOS:000176296700065. |
| [24] | Fayzullayev, N. I.; Muradov, K. M.; Fayzullaev, O. O. Catalytic Liquid-Phase Hydrochlorination of Acetylene. In Abstracts of Papers, 223rd ACS National Meeting, Orlando, FL, United States, April 7-11, 2002; 2002; pp U25–U25. https://www.webofscience.com/wos/woscc/full-record/WOS:000176296700081. |
| [25] | Fayzullaev, N. I.; Muradov, K. M.; Fayzullaev, O. O.; Umirzakov, R. R. Some Problems of Kinetics of Process of Steamphase Hydration of Acetylene. In Abstracts of Papers of the American Chemical Society; 2002; pp U22–U22. https://www.webofscience.com/wos/woscc/full-record/WOS:000176296700064. |
| [26] | Fayzullaev, O. O., Muradov, K. M., & Fayzullayev, N. I. Mechanism of reaction of oxidative condensation of methane. In Abstracts of Papers of the American Chemical Society. 2002; Vol. 223, pp. U27-U27. 1155 16TH ST, NW, Washington, DC 20036 USA: Amer Chemical Soc. https://www.webofscience.com/wos/woscc/full-record/WOS:000176296700095. |
| [27] | Parsaee, Faeze, Normurot Fayzullaev, Maadh Fawzi Nassar, Baraa Abd Alreda, HassabAlla MA Mahmoud, Anmar Ghanim Taki, and Monireh Faraji. "Co-Fe dual-atom isolated in N-doped graphydine as an efficient sulfur conversion catalyst in Li-S batteries." Journal of Alloys and Compounds 988 (2024): 174136. |
| [28] | Ibraheem Shelash Al-Hawary, Sulieman, Raed Obaid Saleh, Ahmed Rafiq AlBajalan, Normurot Fayzullaev, Mohammed Alshuhri, Saad Hayif Jasim Ali, Ahmed Alawadi, Mohammed Abed Jawad, Salim B. Alsaadi, and Maryam Sadat Ghorayshi Nejad. "Synthesis of N, N′-alkylidene bisamides and Suzuki–Miyaura coupling reaction derivatives with Pd organometallic catalyst anchored to channels of mesoporous silica MCM-41." Scientific Reports 14, no. 1 (2024): 7688. |
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML