-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
International Journal of Materials and Chemistry
p-ISSN: 2166-5346 e-ISSN: 2166-5354
2024; 14(6): 109-111
doi:10.5923/j.ijmc.20241406.03
Received: Dec. 12, 2024; Accepted: Dec. 28, 2024; Published: Dec. 31, 2024

Development of a New Sorption- Spectrophotometric Method for Determining the Ion Fe(III) Using Immobilized Methylthymol Blue
N. F. Davronova
Phd Student, Tashkent Institute of Chemical Technology, Uzbekistan
Correspondence to: N. F. Davronova, Phd Student, Tashkent Institute of Chemical Technology, Uzbekistan.
Copyright © 2024 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

An accelerated method for sorption-spectroscopic determination of iron ions in natural wastewater using immobilized methylthymol blue with improved metrological indices has been developed. Conditions for immobilization, complexation and detection of iron ions have been optimized. It has been established that the optimal buffer at pH 2.5 is a universal buffer solution. The developed method has been used in the analysis of drinking water with a detection limit of 0.1 μg/l.
Keywords: Analytical reagent, Iron ion, Methylthymol blue, Immobilization, Asorption-spectrophotometric
Cite this paper: N. F. Davronova, Development of a New Sorption- Spectrophotometric Method for Determining the Ion Fe(III) Using Immobilized Methylthymol Blue, International Journal of Materials and Chemistry, Vol. 14 No. 6, 2024, pp. 109-111. doi: 10.5923/j.ijmc.20241406.03.
1. Introduction
- Iron is one of the microelements important for the human body. Deficiency of iron in the human body causes various vascular diseases [1]. However, it is worth noting that excess iron in the human body causes damage to the liver, pancreas and myocardium - the development of hemochromatosis [2]. Iron plays an important role in the development of the ecosystem, agriculture and industry, but at the same time it is a pollutant of water bodies [3]. Heavy metals in drinking water pose a threat to human health. Therefore, it is necessary to determine and control the amount of iron ions in environmental objects. A simple and rapid method of solid-phase spectrophotometry for determining free iron (III) in environmental and biological samples is proposed [4]. Disodium salt of 1-nitroso-2-naphthol-3,6-disulfonic acid (Nitroso R salt) and hexadecyltrimethylammonium bromide were used as ligands [5]. A flow-injection spectrophotometric method for determining the amount of dissolved and total iron in tap and natural waters has been developed. The catalytic effect on the oxidation of N,N-dimethyl-p-phenylenediamine was determined in samples of natural waters [6]. The spectrophotometric method for determining iron ions using various ligands does not allow for wide application due to its selectivity and low sensitivity. Iron (III) ion can be determined by atomic absorption spectrometry using immobilized sulfosalicylic acid [7], immobilization of sulfarsazene ligand on polyacrylonitrile-modified polyethylenepolyamine fiber [8]. Inductively coupled plasma mass spectrometry (ICP MS) has been used to determine iron and iodine elements in watermelon seeds [9], iron and many microelements in the plant, leaves and seeds of the akkhangal plant [10], and 44 macro- and microelements in the flowers of Tanacetum vulgare [11]. However, these methods require expensive equipment. Sorption-spectrophotometric methods for determining heavy and toxic metals using immobilized organic reagents have also been developed. For example, immobilized bismuth ion xylene orange [13], mercury(II) ion immobilized on PPA1 fiber [14], immobilized disodium reagent 1,8-dioxonaphthalene-3,6-disulfonic acid for detection of chromium ions [15], manganese ions immobilized with alizarin. Methods of sorption-spectroscopic detection using -3-methylamino-N,N-diacetic acid have been developed [16].At present, one of the widely developed methods is absorption spectrophotometry. This is an improved version of the spectrophotometric method, since the sensitivity of the spectrophotometric method is 10-6-10-8. If the sample amount is small, it should be evaporated without preliminary concentration. The sorption-spectroscopic method can be used to determine trace amounts of metal directly using an organic reagent immobilized on a fiber. This modified method includes several processes: concentration, determination directly in the solid phase, without separating the metal ion [17].
2. Methods and Materials
- 0.5585 g of high-purity metallic iron is dissolved in 10 ml of a mixture of hydrochloric and nitric acids (3:1), diluted to 50 ml with water and boiled until nitrogen oxides and 5585 mg/ml of Fe3+ chlorides are removed. A standard nitrate solution is formed [18]. Buffer solutions [19] were prepared based on literature data. To prepare a 0.01 M methylthymol blue solution, 0.76085 g of the reagent powder is measured out on an analytical balance and distilled water is added to a 100 ml flask. To immobilize the selected fiber, 0.2 g is weighed on an analytical balance and activated with a 0.1 N HCl solution (24 hours). After activation, it is washed with distilled water to pH = 7. The fiber is kept wet in a Petri dish and stirred with a stirrer by adding 10 ml of 0.01 M solution of our reagent [17]. ABS 120-4N analytical scales (China) were used to measure the samples. The reagent environment was measured using a pH meter "PHS-3E" (China). The reflectance of immobilized fibers was measured using an X-Rite Eye one pro spectrophotometer (380-730 nm), and the light absorption of reactive solutions was measured using an EMC-30PC-UV Spectrophotometer and "UV-5100 UV VIS".
3. Results and Discussion
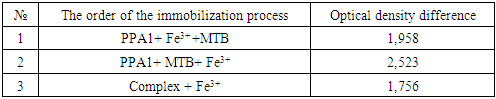
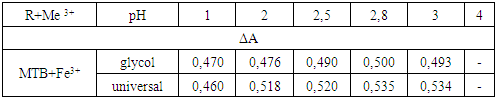
- A sorbent for immobilization of an organic reagent was selected and the order of adding components to the immobilization process was determined. Optimal conditions for the formation of a complex of the immobilized organic reagent methylthymol blue (MTB) with Fe3+ were established. The research work studied the time dependences of complex formation, optimal pH values and optimal buffer solutions, the order of adding buffer solutions during the formation of complex compounds and other features.When choosing the optimal conditions for immobilization of organic reagents on fibrous sorbents, 0.2000 g were taken from several types of sorbents on an analytical scale and activated with a standard solution of 0.1 M hydrochloric acid for 24 hours, then washed with distilled water and stored in a special Petri dish (in a wet state). For the analysis, CMA1, PPD, PPF and PPA1 fibers were selected, 0.2000 g of the selected fibrous sorbents were placed in a beaker, 10 ml of 0.1% solution of our methylthymol blue reagent was added and stirred for 5-10 minutes. The reflection spectrum of the immobilized fibers was measured on an X-rite one spectrophotometer (Switzerland).
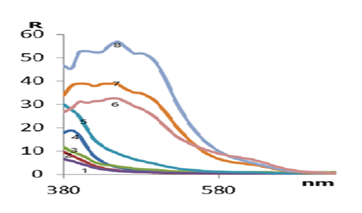 | Figure 1. Reflectance spectrum of fibers immobilized with methylthymol blue reagent. (1-CMA; 2-PPD; 3-PPF; 4-PPA; 5-Imm.CMA; 6-ImmPPD; 7-Imm PPF; 8-Imm PPA) |
|
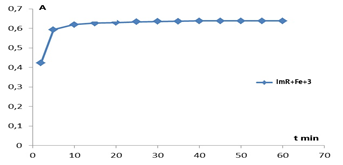 | Figure 2. Dependence of complex formation of immobilized MTB with iron (III) ion on time |
 | Figure 3. Dependence of the formation of a complex of immobilized MTB with the Fe3+ ion on the pH of the environment |
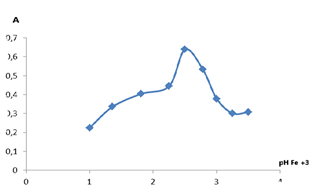 | Figure 4. Optical density of iron complex formation at different pH values |
|
4. Conclusions
- In this research work, the optimal sorbent PPA1-fiber for immobilization of the methylthymol blue reagent was selected. A sorption-spectroscopic method for determining the iron (III) ion with immobilized MTS was developed. The optimal conditions for the formation of the complex compound were studied: dependence on time, pH of the medium, buffer solution and the order of adding the components.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML