N. B. Eshmamatova1, L. M. Qurbanova2, A. M. Khamidov3
1National University of Uzbekistan, Tashkent, Uzbekistan
2Jizzakh Polytechnic Institute, Jizzakh, Uzbekistan
3Turin Polytechnic University in Tashkent, Tashkent, Uzbekistan
Correspondence to: N. B. Eshmamatova, National University of Uzbekistan, Tashkent, Uzbekistan.
| Email: |  |
Copyright © 2024 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Abstract
In article the results of electrochemical and gravimetrical investigations by the influence of new elaborated oligomeric salts on the base of some organic compounds on the corrosion behavior of steel samples (St.3, 45) in different mediums are presented. It was shown that protective effect of investigated oligomeric salts has been increased with increasing their molecular mass and hydrocarbon radicals. The influence of the experiments duration and also some other factors on the protective effect of the investigated oligomers have been discussed.
Keywords:
Inhibitors, Steel corrosion, Mechanism of inhibition, Adsorption, Polarization curves, Polarization resistance, Hydropholical, Synergism, Electrodonoral, Acetamide, Formamide, Aminoethanol, Melamine phosphate, Melamine glycerate phosphate, Dimethylolhexamethylenediamine phosphate
Cite this paper: N. B. Eshmamatova, L. M. Qurbanova, A. M. Khamidov, Inhibition of Metal Corrosion Based on Nitrogen and Phosphorus Containing Organic and Oligomeric Salts, International Journal of Materials and Chemistry, Vol. 14 No. 5, 2024, pp. 86-96. doi: 10.5923/j.ijmc.20241405.02.
1. Introduction
Carrying out of purpose investigations by increasing of quality of metals and their effective using is important in all world and at this special attention is devoted to following tasks: using of water-soluble oligomer and polymeric inhibitors; fighting with salt-deposits, determination of optimal conditions of inhibition of metals corrosion, synthesis of water-soluble inhibitors on the base of nitrogen and phosphorous - containing compounds effectively protecting black and non-ferrous metals from corrosion in deferent mediums, investigation of mechanisms of their action, determination of different physic-chemical particulates of their action [1]. The high pollution of environment and hard regimes of exploitation of the technological equipment of oil-gaseous branch have caused great damage from the corrosion which in industrial development contrives has achieved about 10% of the combined profit. At the exploitation of cooling systems of circulating water-supply in without through high regime the brevity of using of circulating waters has increased what has caused the increasing of their mineralization [2]. Aliphatic, aromatic and heterocyclic amides, amines, hinolines and some other compounds are the most investigated in this plane. In spite of the wide spectrum of investigations in this field some questions have remain practically not enough investigated [3]. Anodic protection by the passivating inhibitors has based on the fact that in process of the reduction the current has appeared which enough to transition metals in passive state. Some salts of Fe3+ – nitrates, dichromate’s and some others can be used as inhibitors. Application of such inhibitors has allowed to protect metals in hard accessible places – cracks and clearances; it is necessary to note that their short ate is a contamination of technological medium [4]. Inhibitors can change the rate of corrosion processes if they can to influence on the kinetics of the electrochemical reactions which have caused the corrosion. At present time the most elaborated are physic – chemical, electro-chemical and some others method in investigation of inhibitors of corrosion, but development of technologies demands to study mechanism of inhibitoral action of organic compounds on processes of corrosion and increasing of steel protection [5]. In oil production and oil refining conditions, metal equipment is exposed to very aggressive environments. The aggressive properties of field environments are due by the high mineralization of oil field waters and the presence of dissolved gases in them – hydrogen sulfide, carbon dioxide, hydrogen chloride, oxygen. The most common and problematic for the oil industry now are: carbon dioxide corrosion, hydrogen sulfide corrosion, hydrogen embrittlement, etc. [6]. Organic compounds are predominantly used as inhibitors, less often than inorganic ones. Mixtures of substances, which in most cases are industrial waste, by-products or easily accessible components are modified to varying degrees to give them the necessary properties are widely used. This work is devoted to heterorganic compounds, which are secondary and tertiary amines and their derivatives, as well as their salts [7]. Organic compounds containing double and triple bonds are utilized as corrosion inhibitors across various industrial applications owing to their capacity to form protective films on metal surfaces, thereby preventing or decelerating the corrosion process [8,9]. Sodium polyphosphate is used to soften water, and sodium tripolyphosphate and other polyphosphates can form stable complex compounds in a short time in the temperature range of 20-40°C [10]. The objects of study are nitrogen and phosphorus-containing low-molecular organic compounds such as acetamide, formamide, aminoethanol, and also synthesized salts of melamine phosphate, melamine glycerate phosphate and dimethylolhexamethylenediamine phosphate.
2. Materials and Methods
Electrochemical (Corrtest: Potentiostat/Galvanostat), gravimetrical IR-spectroscopic (IR-spectrometer EN 1407) and X-ray diffraction (XRD) using a X-ray diffractometer (DRON-05) analysis was carried out to determine isomorphous compounds, X-ray diffraction patterns, parameters and possible space group of new compounds.Corrosion inhibitor (AKI-1): acetamide CH3CONH2 is the amide of acetic acid. Acetamide is a needle-shaped crystal with a mousy odour, a colourless or light grey mineral that diffuses in moist air. It is highly soluble in water, hot alcohols and number of organic solvents, and practically insoluble in benzene and ether. Evaporates in sunlight. Molar mass 59.07 g/mol, density ρ=1.16 g/cm³; Tboiling.=221.2°C; Тmelting.=79-81°C. Corrosion inhibitor (AKI-2): formamide is the amide of formic acid, the simplest carboxamide - the first member of the homologous series of amides of carboxylic acids. Transparent, viscous liquid, molar mass 45.04 g/mol, density ρ=1.13 g/cm³; Тmelting.=2-3°C; Tboiling.=210°C. Traditional name-formamide. Chemical formula CH3NO, rational form HCONH2. At atmospheric pressure and temperatures above 160°C formamide decomposes with formation of form ammonia and carbon monoxide.Corrosion inhibitor (AKI-3): 2-aminoethanol HO-CH2CH2-NH2 (glycinol, common name calamine) is an organic substance of the amino alcohol class, is a primary amine and a primary alcohol. Also it is called monoethanolamide. Chemical formula C2H7NO, molar mass 61.08 g/mol, density ρ=1.012 g/cm³; Tboiling=170°C; Тmelting.=10.3°C. Viscous oily liquid, has a slight amine odor, miscible with water in all respects. Well soluble in ethanol, benzene, chloroform.Corrosion inhibitor (AKI-4): based on melamine and phosphoric acid. Melamine (0.1 M) and phosphoric acid (0.1 M) are loaded into a round-bottomed flask equipped with a mechanical stirrer and mixed until completely dissolved and a homogeneous mass is obtained. Then the temperature rises to 60-70°C, the reaction is carried out until the sediment is completely separated, which is dried in the open air. Corrosion inhibitor (AKI-5): based on melamine, phosphoric acid and glycerin: A 0.1M solutions of melamine and phosphoric acid with glycerin were poured into a 250 ml round-bottom flask equipped with mechanical stirrer. Next a mechanical stirrer was turned on and the reaction mixture was heated to 70°C. The resulting salt has a light purple color and is a crystalline powder. Corrosion inhibitor (AKI-6): based on hexamethylenediamine adduct phosphate: HMDA (0.1M) and phosphoric acid (0.1M) are loaded into a three-neck flask equipped with mechanical stirrer and mixed until completely dissolution with obtaining homogeneous mass. Then the temperature was raised to 70-90°C, the reaction was carried out until the precipitate is completely separated, which than is dried in the open air:Corrosion inhibitor (AKI-7): based on formaldehyde and hexamethylenediamine: Formalin was poured into a 250 ml round-bottom flask equipped with a mechanical stirrer and neutralized by 25% aqueous ammonia solution to pH=7 (universal indicator). Next, a mechanical stirrer was turned on, hexamethylenediamine was added, and the reaction mixture was heated to 60°C. The reaction mixture was kept at this temperature for 40-60 minutes and then cooled to 40-45°C. The cooled mass was poured into a device for distillation under vacuum, and the water was distilled off at a residual pressure of 60 mmHg. and temperature 60°C. The resulting porous mass was dried in a vacuum oven at 40°C until a solid product was formed, the yield of which was 87-92% from theoretical. Corrosion inhibitor (AKI-8) based on formaldehyde and hexamethylenediamine with phosphoric acid: Formalin was poured into 250 ml round-bottom flask equipped with a mechanical stirrer and neutralized with 25% aqueous ammonia solution to pH=7 (universal indicator). Next a mechanical stirrer was turned on, hexamethylenediamine was added and the reaction mixture was heated to 60°C, gradually mixing with phosphoric acid. The resulting mass was dried in a vacuum oven at 40°C, the resulting salt has a light pink color. Dimethylolhexamethylenediamine with acids forms salt-like compounds such as dimethylolhexamethylenediamine phosphate, which are also highly soluble in water. Dimethylolhexamethylenediamine phosphates in dilute solutions in neutral, slightly acidic and slightly alkaline mediums at ordinary temperatures have a linear structure with a degree of polymerization from 7 to 14, i.e. they are oligomers with a molecular mass about 1750-3500.
3. Results and Discussion
The objects of the study were nitrogen - and phosphorus-containing organic compounds. Investigation of the corrosion behavior of steel (St. 3, St. 45) was carried out on samples in the form of plates. St. 3 - %: Fe=98.36; C=0.20; Mn=0.50; Si=0.15; P=0.04; S=0.05; Cr=0.30; Ni=0.20; Cu=0.20. St. 45 - %: Fe=97; C=0.42; Mn=0.50; Si=0.17; P=0.035; As=0.08; S=0.04; Cr=0.25; Ni=0.25; Cu=0.25. The effect of salts, medium and inhibitors on the corrosion behavior of steel samples was determined by electrochemical methods and gravimetrically by the loss of sample mass after corrosion tests. The purpose of this experiment is to monitor the open circuit potential (or free corrosion potential, the difference in potential as a function of time. The experiment can be carried out for a fixed duration or until a certain potential is reached. Electrochemical methods (Open Circuit Potential) Platinum plate electrode (CS911), Ag/AgCl reference electrode (CS901) were used.With the methods polarization curves, it is necessary to measure the passivation curves of metals in a certain medium. It will create a layer of passivation film on the surface of these metals when their potential is relatively positive. At this point, it changes with the flow of a small passivation of current. To evaluate their anticorrosion performance, it is necessary to determine the potentials cracking and protection. Therefore, a passivation curvers were constructed.Investigations the molecular - dynamical and quant-chemical characteristics of synthesized organically oligomer compounds h,ave allowed to obtain information about their structures and by this reason characteristic have a self – depended interest from the point of view of physical chemistry. Also knowledge of geometry of molecules is necessary for calculations the heats of formation, heat effects and energy of activation of reactions. By this reason electronic densities and distribution of charges in molecules of compounds presented in table 1 and also their structures were determined by semiemperical quanta-chemical method MNDO. As example results of investigation of electronic structure and also structure of inhibitor AKI-6 (Fig.1.) are presented from which it is shown that the most electronic density has placed on both pyrimidine rings.Тable 1. Used organic and oligomeric inhibitors
 |
| |
|
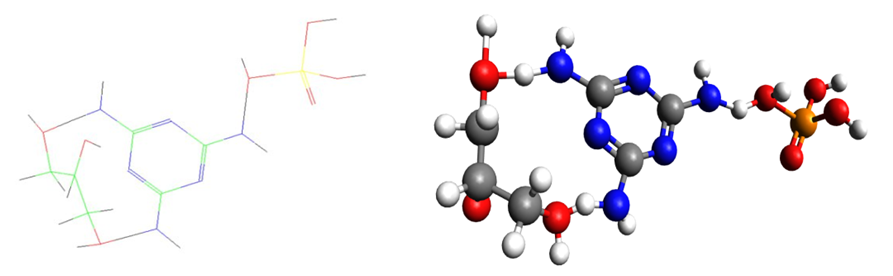 | Figure 1. Structure of AKI-5 molecules and it is ball-pivotal model |
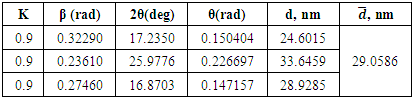
This molecule owing to this can to manufacture electrodoner properties according to electro accepter grapes or to be intermediate which has formed π complexes [11]. It was shown that oxygen atoms of the hydroxyl groups have the most negative charges: -0.811; -0.843 owing to which they can participate in reaction of substitution with different cations of metals or some positive charged groups. These molecules have possessed by acid properties and at dissociation have formed protons and complex anions which at corrosion of metallic surface have formed compounds with positive charged atoms of metals (in base with iron atoms). Also owing to large volume these molecules have covered some surface of metallic constructions. Above-mentioned factors have caused inhibition properties of this type of inhibitors at acid corrosion of metallic surfaces. X-rays are electromagnetic waves with wavelengths in the range 0.01-10 nm. On the short-wave side they are adjacent to -rays (wavelengths less than 0.1 nm), on the long-wave side with ultraviolet (wavelengths approximately 10-380 nm). The size and shape of a cell are determined by the size, shape and relative position of its constituent particles. The geometry of the crystal is described by the dimensions of the unit cell, characterized by the length of its three non-parallel edges (a0, b0, c0) in nanometers and the angles between them (α, β, γ). The X-ray structural analysis method has been applied to collagen research in two ways: 1) high-angle study and 2) low-angle study. Both methods are based on the same principle - diffraction and interference of X-rays reflected from internal planes in the crystal.It is known, that crystal is built from a more or less complex groups of atoms - a motif unit, which is regularly repeated in three dimensions by linear transfers and translations. And these complications in the interpretation of X-ray diffraction patterns of polymers (Fig.2.) are obviously due to the difference in structure and structure of the latter from low molecular mass of crystalline substances. λ=2d sin θ where λ is the wavelength of X-rays; d-distance between planes; θ is the “angle of reflection” of X-rays.Table 2. Changes in melamine phosphate parameters in unit cells
 |
| |
|
Regarding continuous diffuse scattering near the central spot, there are indications without a detailed analysis of this phenomenon.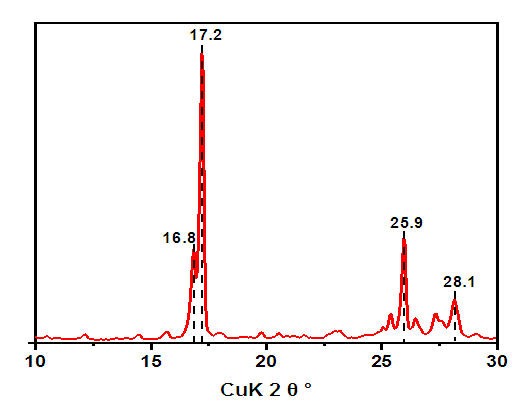 | Figure 2. XRD pattern of melamine phosphate (AKI-4), λ=0.15406 nm |
Basically, almost all works are devoted to discrete scattering at small angles. These atomic groups, representing lattice sites, are capable of scattering X-ray radiation incident on them only under certain conditions.Table 3. Changes in parameters of melamine glycerate phosphatein unit cells (AKI-5)
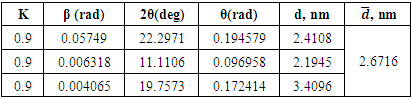 |
| |
|
In the process of performing X-ray analysis of compounds, the greatest difficulties arise in interpreting obtained data (Fig.3).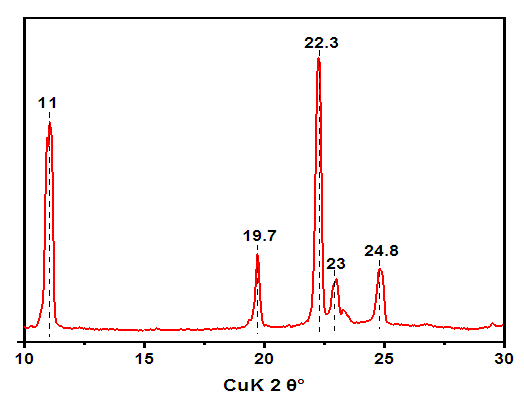 | Figure 3. XRD pattern of melamine glycerate phosphate (AKI-5), λ=0.15406 nm |
If a parallel monochromatic beam of X-rays is passed through a crystal, then the rays emerging from it will have diffraction maxima at angles.Table 4. Changes in the parameters of phosphate hexamethylenediamine (AKI-6) in unit cells
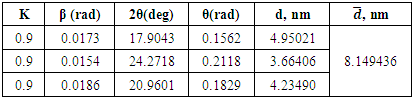 |
| |
|
Atoms in a crystal are interconnected by characteristic symmetry relationships, according to which these crystals are divided into classes (Fig.4).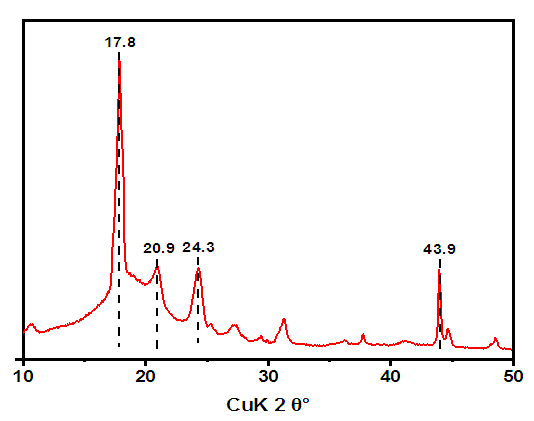 | Figure 4. XRD pattern of hexamethylenediamine phosphate (AKI-6), λ=0.15406 nm |
The interpretation of the results obtained from radiographic examination at small angles is different. Detectable when photographed at high angles must be part of a large period detected when photographed at low angles.Table 5. Changes in parameters of hexamethylenediamine formaldehyde (AKI-7) in unit cells
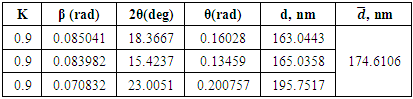 |
| |
|
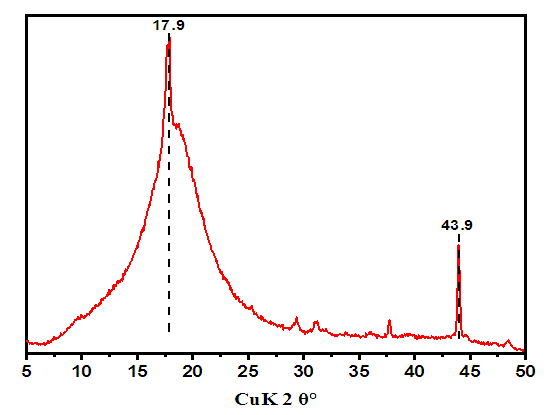 | Figure 5. XRD pattern of hexamethylenediamine formaldehyde (AKI-7), λ=0.15406nm |
These limiting values were obtained in cameras capable of detecting periods of 100.0 nm. Then, based on the found values, the cell parameters are set (Fig.5). However, the task of interpreting X-ray diffraction patterns becomes significantly more complicated when studying structures related to single-axle systems.Table 6. Changes in the parameters of dimethylolhexamethylenediamine phosphate (AKI-8)
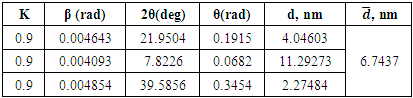 |
| |
|
As you know, a crystal is a solid substance consisting of orderly arranged atoms or atomic groups that periodically are repeated in three directions. It is formed from many elementary cells, tightly adjacent to each other on all sides (Fig.6). As is known, for this purpose, the distances between the conjugate lines on the radiograph are first measured and the values of θ are determined from them [12].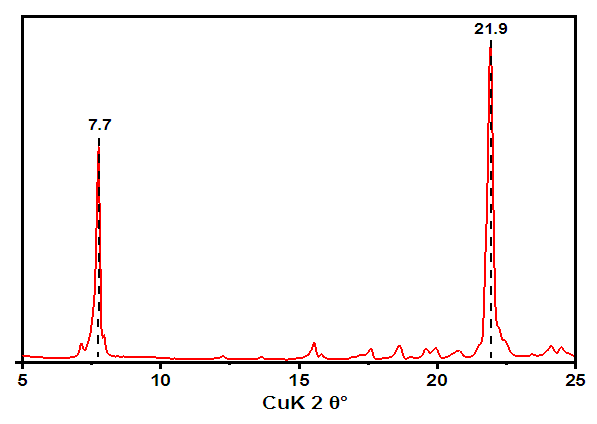 | Figure 6. XRD pattern of dimethylolhexamethylenediamine phosphate (AKI-8), λ =0.15406 nm |
IR spectrums of the obtained salts and comparison of inhibitory connection properties. Comparative results of a study of the inhibitory properties of the compounds melamine phosphate, HMDA phosphate, melamine phosphate glycerate, hexamethylenediamine formaldehyde are presented, which showed that their protective effect is due by formation of an adsorption layers on the metal surface.For comparison the IR spectrum (Fig.7) of melamine was obtained, in this structure new absorption bands appear in the range 3468.01-3417.86 cm-1, characteristic to NH2 groups. In the range 2821.86-2662.98 cm-1 CH groups appear, at 1625 cm-1 bending vibrations of the NH2 group, the band at 1533.41 cm-1 shows quadrant vibrations of the 1.3.5-triazine ring, in the region of 1431.18 cm-1 half ring vibration of the 1.3.5-triazine ring, 1193.94 cm-1 band corresponds to stretching vibrations associated with the C-N group and is usually associated with the main stretching vibrations of heteroatom rings, at 1022.27 cm-1 vibrations of the C-N group primary amines with tertiary carbons. It is assumed that the inhibitory effect is stronger in relation to some metals of inhibitors containing functional groups -NH2, =NH, CN, N=O. The spectrum of the inhibitor contains two components corresponding, respectively, to the covalent bonds P-O and P=O which are not involved in the coordination with d-metal atom.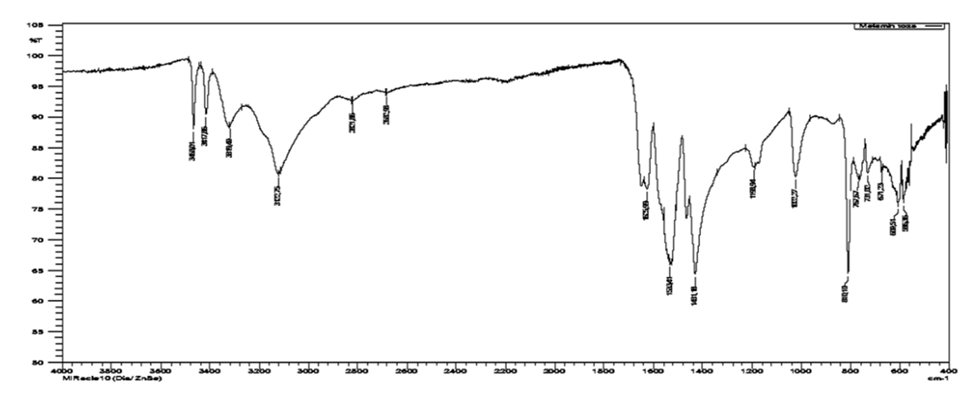 | Figure 7. IR spectrum of melamine |
In the IR spectrum (Fig.8.) of melamine phosphate (AKI-4) the broad peak at 3331.07 cm-1 is caused by the vibrations of the OH group; 3093.82 cm-1 is attributed to the stretching vibrations of NH3+; 1379.10 cm-1 corresponds to the C-N absorption peak in the thiazine structure; 1271.09 cm-1-to the absorption peak of the P=O group; 1056.99 cm-1 for the vibrational absorption of the P-O group in P-OH. 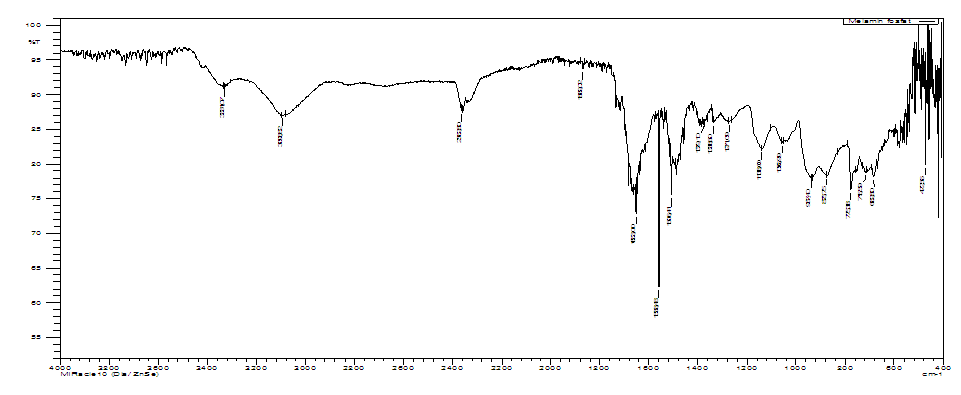 | Figure 8. IR spectrum of melamine phosphate (AKI-4) |
At protection by inhibitors in aqueous systems, a protective film is formed on the surface of low-carbon steel, mainly consisting of complexes with iron cations [13]. The region 3373 cm-1; -CH2- groups give asymmetric and symmetric (Fig.9) broad vibrations in the regions 2941-2885 cm-1; stretching vibrations of -C-O groups are observed in the region of 1725-1671 cm-1; vibrations indicating the location of P-O-P groups are located in the region 976-927 cm-1; CH groups appear in the range 775-713 cm-1 and 680 cm-1; and in range 579-509 cm-1 there is absorption maximum corresponding to the -NNN bond.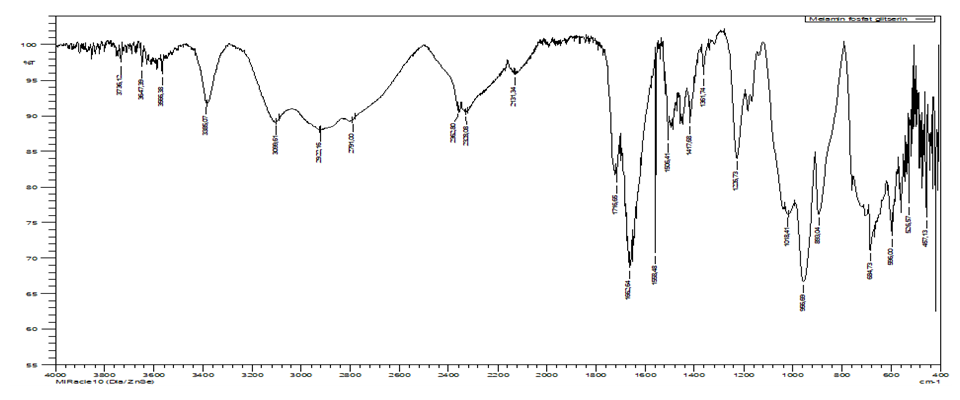 | Figure 9. IR spectrum of melamine phosphate glycerate (AKI-5) |
In the IR spectrum of hexamethylenediamine phosphate there are 609.51, 671.23, 812.03, 877.61 and 916.19 cm-1 frequencies with average intensity related to non-plane deformation vibrations corresponding to torsional -CH2 group bands which are quite characteristic to this functional group (Fig.10). 1558-1683 cm-1 -NH2 plane bending vibrations corresponding to scissor vibrations like -CH2; bands 1004-1126 refer to R-NH2; in the region 1236 cm-1 there are flat band of average height, belonging to the -OH group; the bands in range 1361-1319 cm-1 are attributed to the –P-O group. The main one is that inhibition is due to a screening effect. In the case of AKI-6 inhibitor, adsorption of HMDA phosphate is possible through the nitrogen atom of the amino group, which has a lone pair of electrons [14].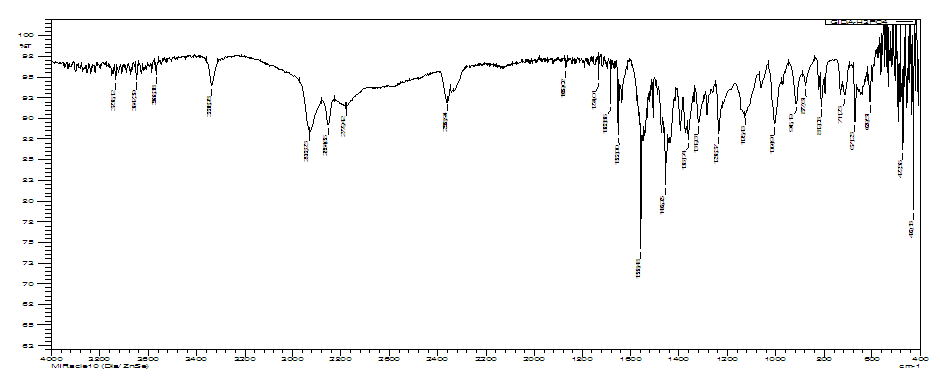 | Figure 10. IR spectrum of hexamethylenediamine phosphate (AKI-6) |
Dimethylolhexamethylenediamine exhibits absorption bands in the range 3732–3566.38 for the -NH2 group, 1398 for C-N, 1163.08 O-C- (Fig.11). In the case of –CH2–CO- bonds they appear in the form of strong narrow bands in the region 1473-1440 cm-1, 2933.73-2846.93 cm-1 νas there are asymmetric bands of CH2 group, 1255.66 cm-1 oberon band can be seen attributed to absorbited CH2 group.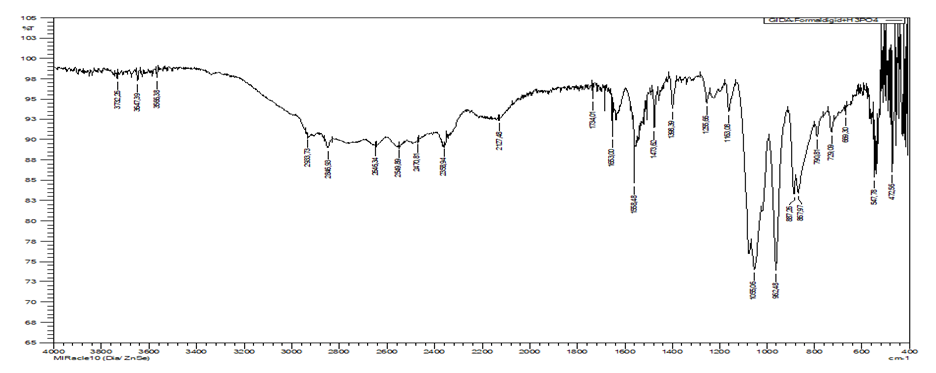 | Figure 11. IR spectrum of dimethylolhexamethylenediamine (AKI-7) |
In circulation systems oligomer inhibitors can be used to prevent corrosion, owing to promotion the deposition of various crystalline layers and presentation the occurrence of pitting corrosion [15]. The pH value should be maintained at 6.5-7.0 or even slightly lower. Based on the data obtained from corrosion experiments, it was established that in circulating waters with a high salt content (F-2) there are not only aggressive anions (ClO-, SO42-) but also calcium and magnesium ions, participated in the formation of protective films on the metal surface and decreasing corrosion aggressiveness [16]. The protective film of the inhibitor on the surface of steel consists of a single component, what indicates on complete involvement of the phosphonate groups of the inhibitor in formation during its adsorption protective film on the metal surface [17]. Thus, the effectiveness of these corrosion inhibitors is due by the presence of two adsorption-active centers and the adsorption of their decomposition products. The carbonyl group is planar, the C and O atoms are in an SP2 hybrid state and differ greatly by electronegativity and, as a result, the bond is very polarized [18]. An example is the action of two depassivators, discovered for the first time on steel and other metals and alloys. The strongest interactions occur with the molecules containing P-O-P; P-O-C; P-H; Р-C; P=O groups and similar strong effect is observed in case of using two-component systems. Results obtained by electrochemical methods on figure is shows the results of study of the open circuit potential for St.45 in a background solution (pH=8.9) of 3%NaCl+5%Na2CO3 in the absence and presence of corrosion inhibitor at concentration 30 mg/l are presented (Fig.12). In general, open-circuit potentials shifted toward more negative values in the presence of the inhibitor. This is due to the orientation of adsorption and the structure of the inhibitor molecule. The metal surface is negatively charged with many functional groups of phosphoric acid contained in the inhibitors.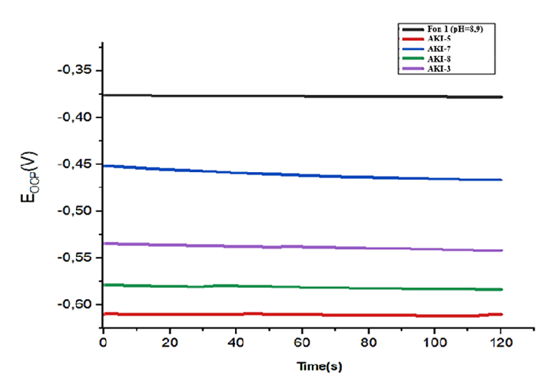 | Figure 12. OCP measurements with background solution Fon-1 with various inhibitors 30 mg/l Fon-1 (1); АKI-5 (2) АKI-7 (3) АKI-8 (4); АKI-3 (5) |
At the same time AKI-5 and AKI-7 are more stable, have the greatest negative value in comparison with other inhibitors, they form an insoluble and protective thin films on the metal surface in a background solution, while the potential above the OCP (open circuit potential) is considered thermodynamic unstable and susceptible to corrosion. These data show that noble potential shifts were observed for all samples, but some of the potentials stabilized faster than others (Fig.13).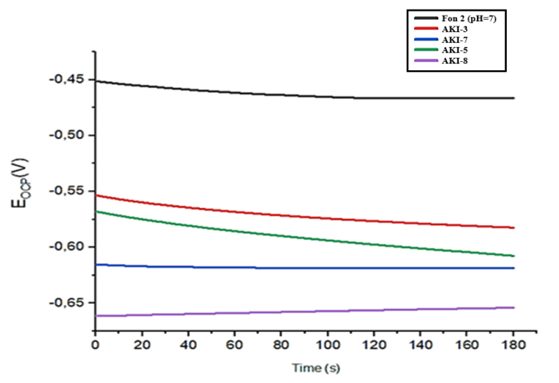 | Figure 13. OCP measurements with background solution Fon-2 pH=7 with different inhibitors 30 mg/l Fon-2(1); АKI-3(2); АKI-7(3); АKI-5 (4); АКI-8(5) |
After immersion in the inhibitor, the final stationary of the sample, Ecorr., is measured. before extraction, the initial mass, final mass and mass loss of each sample, the average corrosion rate calculated from the mass loss, and visual evidence of pitting on the sample after the test. Negative corrosion rates can by result of combination of measurement errors and the formation of a protective film.In this work for the first time was shown that the introduction of oligomeric compounds into electrolytes leads to a decrease in the rate of proton discharge and metal ionization not only in the activation region, but also in the region of limiting currents. A decreasing in the limiting currents clearly indicates on the emergence of additional diffusion restrictions associated with the formation of surface layers, that allowed to conclude that it is necessary to revise the correctness and applicability of existing adsorption theories of inhibitory protection of metals from corrosion. By the degree of reduction in the limiting currents of proton discharge and cathode reduction of oxygen, one can judge both the degree of protection of metals from corrosion destruction and the mechanism of such protection.The results (Fig.14) obtained show that the values of the electrode potential of St.45, with a concentration of 30 mg/l, in the background solution (Fon-1) pH=8.9 are characterized by even more negative values, and the most effective inhibitors are AKI-3 and AKI-8. The most negative electrode potential with inhibitors AKI-5 and AKI-8 was obtained in a background solution (Fon-2) pH=7.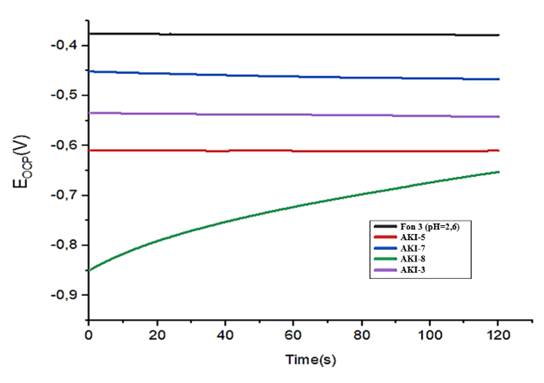 | Figure 14. OCP measurements with background solution Fon-3 pH=2.6 with various inhibitors 30 mg/l Fon-3 (1); АKI-5 (2); АKI-7 (3); АKI-8 (4); АKI-3(5) |
The state of the surface of the samples can be unambiguously judged by the value of the electrode potential. In this regard, open-circuit potential measurements were carried out in parallel with corrosion tests. Potential measurements with a background solution (Fon-3) pH=2.6 with various inhibitors of 30 mg/l determined effective inhibitors AKI-8 and AKI-3.Measuring corrosion rate by methods polarization curves. The most fundamental procedure for the experimental evaluation of icorr. is the Tafel extrapolation. This method requires a linear or Tafel portion of the E versus logiex curve. Potential sweep from approximately ±160 to ±300 mV, relative to Ecorr it is usually required to determine whether a linear portion of at least one decade of current is present so that a reasonably accurate Ecorr. extrapolation can be made potential. With cathode polarization the rate of hydrogen evolution increases but the rate of metal dissolution decreases. Thus, with the help of cathode polarization it is possible to protect the metal from corrosion. Based on the data of polarization measurements near a stationary potential, using linear electroporation of the obtained curves, the corrosion current and the slope angle of the polarization curves of hydrogen evolution and metal dissolution were determined.As indicated, the Tafel curves (Fig.15) are located in more negative and low corrosion current regions in the presence inhibitors than without them, confirming that the amount of chloride and hydrogen ions can decreased in the presence of inhibitors in background solution of pH=8.9 and at this best results were shown by inhibitors AKI-3, AKI-5 and AKI-8 with concentration 30 mg/l. Without inhibitors, these aggressive ions make the metal surface more polarized; Ecorr. is 0.60 V as a result the working electrode made from St.45 steel is destroyed and a lot of corrosion deposits form on it surface.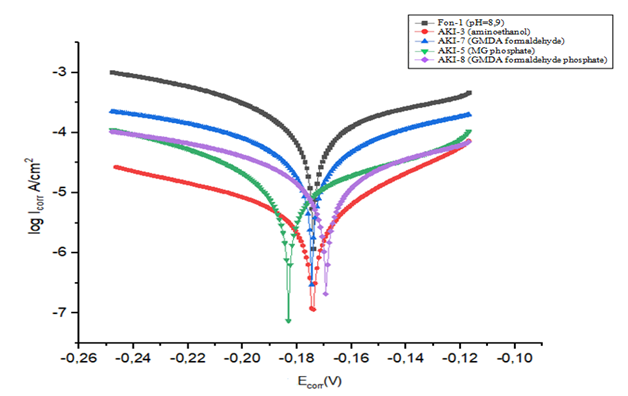 | Figure 15. Polarization curves of a steel electrode in a background solution Fon-1 with various inhibitors АKI-3 (2); АKI-7 (3); АKI-5 (4); АKI-8 (5) |
This effect can be explained by the formation on steel of a thin protective film by melamine glyceride phosphate (AKI-5) and dimethylolhexamethylenedimine phosphate oligomer (AKI-8) which block the surface of the steel and slow down the rate of corrosion destruction. Simultaneously with the change in corrosion potential, a decrease in the corrosion current is observed, that indicated on mixed mechanism of action of the inhibitors (Fig.16).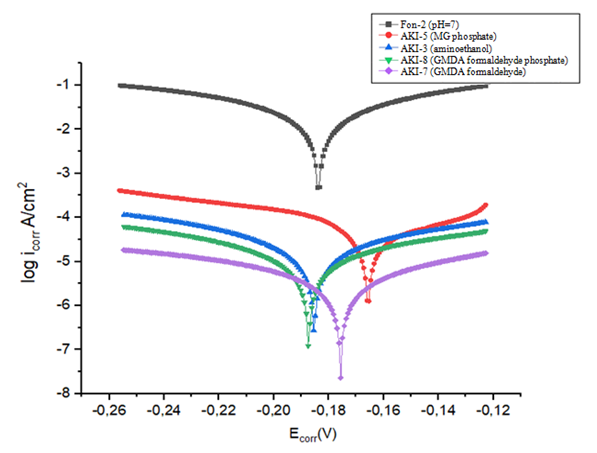 | Figure 16. Polarization curves of a steel electrode in a background solution Fon-2 (1), with various inhibitors АKI-5 (2); АKI-3 (3); АKI-8 (4); АKI-7 (5) |
Sufficiently high corrosion rates were observed in some cases from a passive state [19]. Meanwhile, information about the state of the steel surface during corrosion tests is very important because passivates are effective only in the case of low corrosion rates from a passive state. The state of the surface of the samples can be unambiguously judged by the value of the electrode potential.In order to clarify the effect of additions of various organic inhibitors on the stability and depth of the passive state of St.45 in the background solution, measurements of the electrode potential were carried out over time – every 5 minutes during 25 minutes of exposure of the sample in an aggressive medium (Fig.17).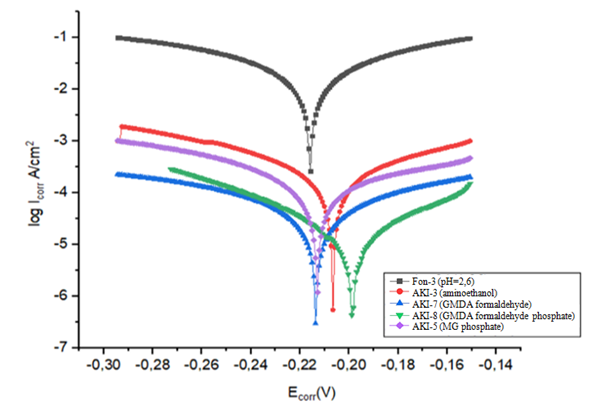 | Figure 17. Polarization curves of a steel electrode in a background solution Fon-3 (1) with various inhibitors АKI-3 (2); АKI-7 (3); АKI-8 (4); АKI-5 (5) |
In the presence of an inhibitor in background solution pH=7, the best results were shown for inhibitors AKI-3, AKI-8 and AKI-7 with a concentration of 30 mg/l. Addition of inhibitors reduced the polarization of the working electrode and blocked the anodic and cathodic reaction. Connecting the electrode to the positive pole allows to shift the potential in a positive direction.Table 7. The effectiveness of inhibitors was determined by the method of polarization curves in solution (Fon-1) pH=8.9 (3%NaCl + 5%Na2CO3) at a temperature 25°C
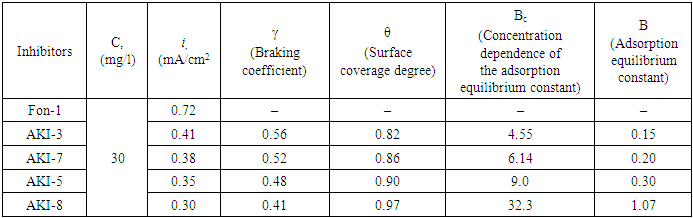 |
| |
|
The obtained data on the degree of filling of the surface with an inhibitor can to determine the adsorption isotherm, the nature of which allows us to obtain valuable information about the properties of the adsorbed substance.By simultaneously determining the current and potential values it is possible to obtain the cathode and anodic polarization curves. Measurements of the electrode potential over time in various background solutions depending on temperature and the presence of additives of PO, –COH and NH, OH- ions showed that without any external influences, over time, the potential of steel tends to improve. A decrease of corrosion current was observed simultaneously with change of corrosion potential and a simultaneous change in corrosion current and potential that means that the inhibitors have acted by mixed mechanism [20]. If the electrode is connected to the negative pole of the current change, then its potential shifts to the negative side. Decreasing of limiting currents clearly indicates on emergence of additional diffusion restrictions associated with the formation of phase surface layers, which in turn leads to the conclusion which was mentioned above that it is necessary to revise the correctness and applicability of existing adsorption theories of inhibitory protection of metals from corrosion.Table 8. Results of gravimetric determination of the protection of St.45 grade metals from corrosion with nitrogen and phosphorus-containing inhibitors (C=30 mg/l) concentrations
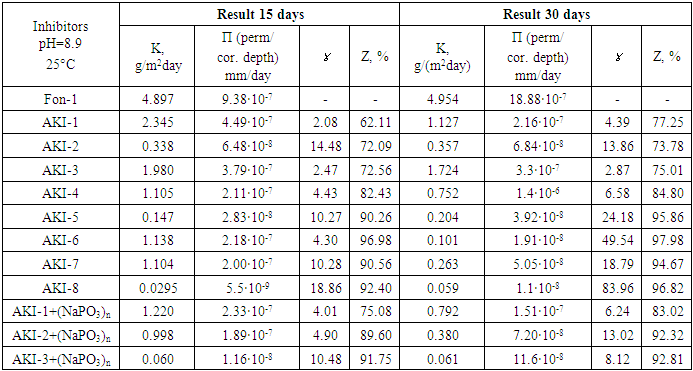 |
| |
|
Thus, in this work it is shown for the first time that the introduction of organic and oligomer compounds into electrolytes leads to decreasing in the rate of proton discharge and metal ionization not only in the activation region, but also in the region of limiting currents. By the degree of reduction in the limiting currents of proton discharge and cathode reduction of oxygen, one can judge both the degree of protection of metals from corrosion destruction and the mechanism of such protection [21]. Results obtained from gravimetric tests: experiments were carried out to determine the corrosion rate of a steel electrode in the presence of the investigated inhibitors at their various ratios in a certain temperature range using the gravimetric method. The results of gravimetric studies and calculations of the corrosion rate and degree of protection by inhibitors based on phosphorus-containing compounds and polyelectrolytes at various ratios and pH have indicated that the most significant results were obtained in alkaline solutions with the addition of sodium polyphosphate (NaPO3)n.
4. Conclusions
Presence in products of corrosion-active compounds is created the actual problem of protection of metals from inner corrosion. Anticorrosion treatment transporting product by inhibitors is important tasks of protection of steel and metallic pipes of different purpose from inner corrosion. Results by investigation of mechanism of protection action of nitrogen and phosphorous inhibitors on the base of H3PO4 and also oligomers and phosphorous-containing adducts has been presented. Phosphorous-containing oligomers as inhibitors of metals corrosion have able to provide effective protection in different aggressive mediums. Results of visual observations have shown that at absence of nitrogen and phosphorous – containing adducts and oligomer salts steel has been undergone to corrosion locally. Investigation phosphorous-containing oligomer inhibitors has shown high effectively of decreasing of progress of steel dissolution in weak–acid and neutral mediums. Distinctive properties of these inhibitors are their low optimal concentration, cheapeners, universal and albescence of toxically. Number of nitrogen and phosphorous – containing adducts on the base of phosphates of thiourea, urea, melamine and hexamethylenediamine and also inhibitors of oligomer type on the base of nitrogen containing organic compounds and phosphoric acid have been synthesized. Molecular dynamical and quantum chemical characteristics of synthesized compounds were determined, mechanism of inhibition by oligomer compounds was exposed and general regularities inheriting to such inhibitors were determined what will to promoted elaboration of new approaches to purposeful synthesis of such inhibitors. On the base of kinetics and thermodynamically investigations it was shown that adsorption properties of inhibitors of oligomer and low-molecular type are different by values of filling degree of electrode surface and constants of adsorption equilibrium.
Authorship Contribution Statement
Eshmamatova Nodira: Methodology, Investigation, Supervision, Writing - review & editing. Qurbanova Latofat: Investigation, Writing - original draft. Khamidov Anvar: Methodology, Investigation, Formal analysis, Software Writing - original draft.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
| [1] | Eshmamatova N.B. Synthesis and physic-chemical properties of oligomeric inhibitors of corrosion on the base of N, P, S containing compounds. 02.00.04 – Physical chemistry. Abstract of doctoral dissertation (DSc). Tashkent. 2016, pp. 82 p. |
| [2] | Eshmamatova N.B. Protection of carbon steel from corrosion by high-effective oligomeric inhibitors. 51th International Scientific conference 2013, pp. 59. |
| [3] | Eshmamatova N.B., Kholikov A.J., Akbarov Kh.I., Tillaev R.S., Asilbekova J.A. Quantitative assessment of the effectiveness of nitrogen - and phosphorus-containing inhibitors according to the results of electrochemical, corrosion and gravimetric studies. J.Uzb.Chem. 2011, pp. 120-123. |
| [4] | Chirkunov A.A. Inhibition of steel corrosion in neutral aqueous media by water-soluble polymers and compositions based on them. Abs. dis. Cand. Chem. sciences. 2007, pp. 27. |
| [5] | Shipiguzov I.A., Kolesova O.V., Vakhrushev V.V., Kazantsev A.L., Poilov V.Z. Lanovetsky S.V., Cherezova L.A. Modern corrosion inhibitors. Herald, 2016, pp. 114. |
| [6] | Islamutdinova A.A., Khaidarova G.R., Dmitriev Yu.K., Sidorov G.M. Synthesis of corrosion inhibitors based on quaternary ammonium compounds and analysis of their protective properties. Oil Chem, 2015, no. 4, pp. 163–168. |
| [7] | Daminev R.R., Islamutdinova A.A., Ivanov A.N., Khamzin I.R. Synthesis of an inhibitory composition to prevent corrosion of oilfield equipment. Butlerov's legacy. 2015, pp. 15. |
| [8] | A.B. Parmanov, S.E. Nurmanov, O.E. Ziyadullaev, L.S. Khoroshko, S.I. Tirkasheva, “Synthesis of vinyl esters of some aromatic carboxylic acids from vinyl acetate,” Azerbaijan Chemical Journal, vol. 2, pp. 53-59, April 2024. |
| [9] | V.M. Muzalevskiy, A.V. Shastin, S.I. Tirkasheva, Z.O.E. iyadullaev, A.B. Parmanov, V.G. Nenajdenko, “CCl4-TMEDA-CuCl-A novel convenient catalytic system for dimerization of terminal acetylenes in mild conditions,” Catalysts, vol. 13, pp. 1330, June 2023. |
| [10] | T. Judit, “Phosphorus-containing corrosion inhibitors: from the beginning till the present time,” Res. Centre for Nat. Sci. vol. 1, pp. 49-68, March 2021. |
| [11] | J.H. Krieger, Computational Chemistry Impact. C&E News. 1997, no. 12, pp. 30. |
| [12] | Y. Abboud, O. Tanane, A. Bouari, Corrosion inhibition of carbon steel in hydrochloric acid solution using pomegranate leave extracts. Corr. Engin. Sci. Techn. 2015, no. 1, pp. 557-565. |
| [13] | N.B. Eshmamatova, А.J. Kholiqov, Kh.I. Akbarov, Synthesis of new oligomers with metal corrosion inhibitor properties. Chem. Chem. Techn. 2012, pp. 39-41. |
| [14] | N.B. Eshmamatova, “Metal corrosion inhibitors based on hexamethylenediamine,” J. Uzbek Chem. vol. 3, pp. 31-33, 2023. |
| [15] | N.B. Eshmamatova, Kh.I. Akbarov, “Inhibitors based on nitrogen and phosphorus-containing oligomeric compounds for the protection of oil and gas equipment,” Rep. Acad. Sci. Repub. Uzb. vol. 2, pp. 45-48, April 2013. |
| [16] | N.B. Eshmamatova, Inhibition of metal corrosion with inhibitors based on organic compounds. Comp. mater, pp. 31-33, March 2013. |
| [17] | S. Chawla, K. Evans, K.M. Sherer, Electrochemical Studies of Open-Circuit Potential Drift of Carbon Steel in Nuclear Waste Simulants. Corr. Conf. & expo. vol. 3, October 2017. |
| [18] | E. Berdimurodov, et al. A gossypol derivative as an efficient corrosion inhibitor for St.2 steel in 1 MHCl+1MKCl: An experimental and theoretical investigation. J. Molecular Liquids. 2021. |
| [19] | N.B. Eshmamatova, Кh.I. Akbarov, Quantitive value of effectively of nitrogen and phosphor-containing inhibitors by the results electrochemical and gravimetrical investigations. Austrian Journal of Technical and Natural Sciences., pp. 132-135, January 2016. |
| [20] | N.B. Eshmamatova, Кh.I. Akbarov, Investigation of anticorrosion properties of some inhibitors systems containing oligomers. Europ. Appl. Sci. pp. 76-79, November 2014. |
| [21] | R. Yusufboy, N. Eshmamatova, Kh. Akbarov, Defense mechanisms and gravimetric estimation of the effectiveness of inhibitors on the base amino- compounds. Universum: Chem. Biol. vol. 2, pp. 20-25, September 2020. |




















 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML






