-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
International Journal of Materials and Chemistry
p-ISSN: 2166-5346 e-ISSN: 2166-5354
2024; 14(5): 81-85
doi:10.5923/j.ijmc.20241405.01
Received: Aug. 21, 2024; Accepted: Sep. 22, 2024; Published: Sep. 28, 2024

Study of Polysaccharides and Their Antimicrobial Effect
Siddikova S. K.1, Eshbekov A. E.1, Maulyanov S. A.1, Azizov D. Z.2
1PhD Student, National University of Uzbekistan, Tashkent, Uzbekistan
2Alfraganus University, Tashkent, Uzbekistan
Correspondence to: Siddikova S. K., PhD Student, National University of Uzbekistan, Tashkent, Uzbekistan.
| Email: |  |
Copyright © 2024 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

The purpose of this work is to study the carbohydrate complex of Cucumis melo crusts, to establish their monosaccharide composition, to determine the physicochemical characteristics and antimicrobial activity. The experimental object of the study: in order to isolate the carbohydrate complex, the bark of Cucumis melo was used, collected during the ripening period in October 2023 on the territory of the Republic of Uzbekistan (Syrdarya region). Monosaccharide composition was determined by using gas chromatography and high performance liquid chromatography, The IR spectra of the samples were taken on an IR Fourier spectrometer. during the experiment, the antimicrobial effect of polysaccharide isolated from melon peel against Serratia marcescens was revealed.
Keywords: Melon (Cucumis melo), Water-soluble polysaccharides (WSPS), Pectin substances (PS), Hemicellulose (HMC), IR spectroscopy, Neutral sugars, Uronic acids
Cite this paper: Siddikova S. K., Eshbekov A. E., Maulyanov S. A., Azizov D. Z., Study of Polysaccharides and Their Antimicrobial Effect, International Journal of Materials and Chemistry, Vol. 14 No. 5, 2024, pp. 81-85. doi: 10.5923/j.ijmc.20241405.01.
1. Introduction
- The pumpkin family (Cucurbitaceae) is a large group of crops with more than 800 species known worldwide. Vegetables from this family have been used for centuries not only for food, but also because of their medicinal value. The most characteristic pumpkin crops are pumpkin, melon and cucumber, which are grown and consumed in many parts of the world. Pumpkin seeds have many health benefits [1]. Melon plants are rich in carotenoids, terpenoids, saponins and phytochemicals. Vegetables from the pumpkin family have a positive effect on human health [2]. So far, a large number of biological activities of polysaccharides extracted from natural sources have been studied, including immunomodulatory activity, antitumor activity, antiviral and antibacterial activity, anti-atherosclerotic ac, anti-inflammatory activity, hepatoprotective activity [3]. Kasaba melon is a good source of vitamin C to strengthen the immune system, vitamin B6 to maintain amino acid levels in the bloodstream and folic acid for the development of red blood cells. Melons also contain potassium to balance body fluid levels, fiber to stimulate the digestive tract, magnesium to regulate nerve and muscle functions, and contain less calcium [4]. Experts believe that Kassaba melons come from Persia and have been grown for thousands of years. In the early centuries, melons were distributed throughout Asia Minor, and were also imported to the rest of Asia and Europe, where they were eaten as a dessert variety [5].The high content of vitamins, carbohydrates, nitrogenous and mineral substances makes melon a very valuable food product for atherosclerosis, colds, diseases of the hematopoiesis and digestion organs, disorders of the cardiovascular system, liver and kidney diseases. The pulp of melon fruits improves intestinal function, has a diuretic and anthelmintic effect, and a decoction of the pulp is used for cosmetic purposes to remove age spots, freckles and acne [6]. Melon has been used as a food and medicinal product since ancient times. Melon peel has a stimulating effect on thyroid function. Experimental studies have shown that aqueous melon extract inhibits platelet aggregation. Chemically, it is known that melon peels contain phenolic compounds-gallic acid, eugenol (4-allyl-2-methoxyphenol) and catechin, but polysaccharides have not been fully studied [7-8].
2. Materials and Methods
- Inactivation of raw materials. 50 g of dried and crushed raw materials of Cucumis melo were treated twice with boiling chloroform for 1 hour at a 1:4 hydromodule to remove coloring and low molecular weight substances. Next, alcohol-soluble sugars were extracted twice with boiling EtOH (82%) (1:4, 1:3). The alcohol extracts were separated by filtration, combined and evaporated to a small volume and analyzed by paper chromatography (PCh) in a 6:4:3 butanol-n-pyridine-water system. Aniline acid phthalate (1) was used to identify the spots to identify hexose and a 5% alcoholic solution of urea - hydrochloric acid (2) to indicate ketosis. Isolation of WSPS. There are many types of polysaccharide extraction methods. Various extraction methods can be selected according to the different properties of polysaccharides, and commonly used polysaccharide extraction methods include hot water extraction, acid extraction, alkaline extraction, ultrasonic extraction, enzyme extraction and microwave extraction [9]. Given the good solubility of polysaccharides in water, the most common method is solvent extraction, including hot water extraction, alkali extraction and acid extraction. To isolate the WSPS, the remainder of the raw material was extracted twice with cold water at room temperature for 1.5 hours at a hydromodule of 1:4, respectively. The extracts were separated by filtration, evaporated to a small volume and precipitated with three times the volume of ethyl alcohol. The precipitate was centrifuged, washed and dehydrated with alcohol. The yield of WSPS is 3.5 g. Further, the remainder of the raw material was extracted twice with water at a temperature of 80-85° for 1.5 hours at a hydromodule of 1:3, 1:2. The extracts were combined, evaporated and precipitated with alcohol. The precipitate was treated as indicated above. Output of WSPS- 2.0 g.Isolation of pectin substances (PS). After isolation of the WSPS, the residue was extracted twice with an equal mixture of 0.5% solutions of oxalic acid and ammonium oxalate at a temperature of 75°C, extraction was carried out with a hydromodule of 1:4, 1:3. The extract was separated by filtration, dialized, evaporated and precipitated with three times the volume of alcohol. The precipitate was treated in the same way as described above. The PS yield is 3.7 g. Isolation of HMC. After isolation of PS, the remainder of the raw material was treated twice with a 5% KOH solution at room temperature, for 1.5-2 hours, with a 1:3 hydromodule. The extracts were separated by filtration, neutralized with CH3COOH, dialized to remove salts, evaporated to a density and precipitated, yield 1.95 g. Complete acid hydrolysis of polysaccharides. The samples of WSPS were hydrolyzed with 1h H2 SO4 at 100°C, 8 hours, PS and HMC 2h H2SO4, 100°C, 20 hours. The hydrolysates were neutralized with barium carbonate, deionized with KU-2(H+) cationite, and evaporated. The qualitative monosaccharide composition of PS was studied by PCh using known witnesses on paper. The quantitative monosaccharide composition of polysaccharides was determined by high-performance liquid chromatography (HPLC). RID-20A detector, aminopropyl column 4.6x250mm, mobile phase acetonitrile water 75:25, 1 ml/min, 40°C [10].The IR spectra of the samples were taken on an IR Fourier spectrometer, System 2000 (Perkin-Elmer) in KBr tablets. The number of scans is 100. Antimicrobial activity of polysaccharides. Before starting the procedure, aqueous solutions of polysaccharides isolated from melon were prepared at a concentration of 100 mg/ml. Conditionally pathogenic bacteria used for testing were grown in a nutrient medium in a thermostat at 37°C for 24 hours. From the grown cultures of opportunistic microorganisms, 0.9% saline solution was diluted until the turbidity index was 107 [11].
3. Results and Discussion
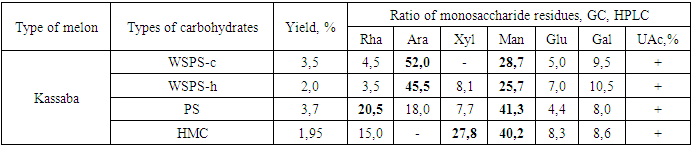
- We have sequentially isolated various polysaccharides from melon crusts: alcohol-soluble sugars (ASS), water-soluble polysaccharides (WSPS), pectin substances (PS), hemicelluloses (HMC). The ASS according to the PCh information (are represented by glucose and fructose. Water-soluble polysaccharides (WSPS) were isolated in two ways: by extraction of raw materials with cold water (WSPS-c), i.e. at room temperature and with hot water at a temperature of 80-90°C (WSPS-h). Pectin substances (PS) were isolated by a mixture of 0.5% solutions of oxalic acid and ammonium oxalate, hemicellulose (HMC) - 5% KOH solution [12]. The yield of polysaccharides and their monosaccharide composition are shown in table 1.
|
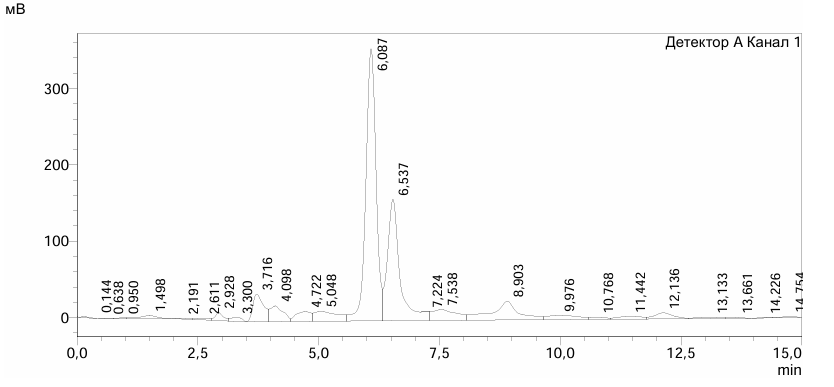 | Figure 1. Chromatogram of WSPS-c |
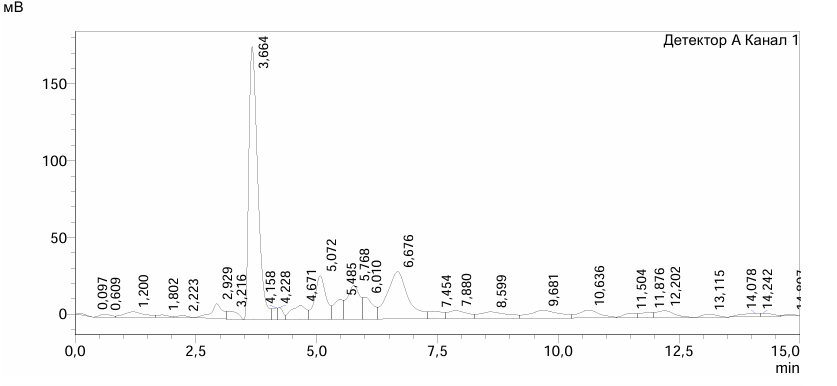 | Figure 2. Chromatogram of PS |
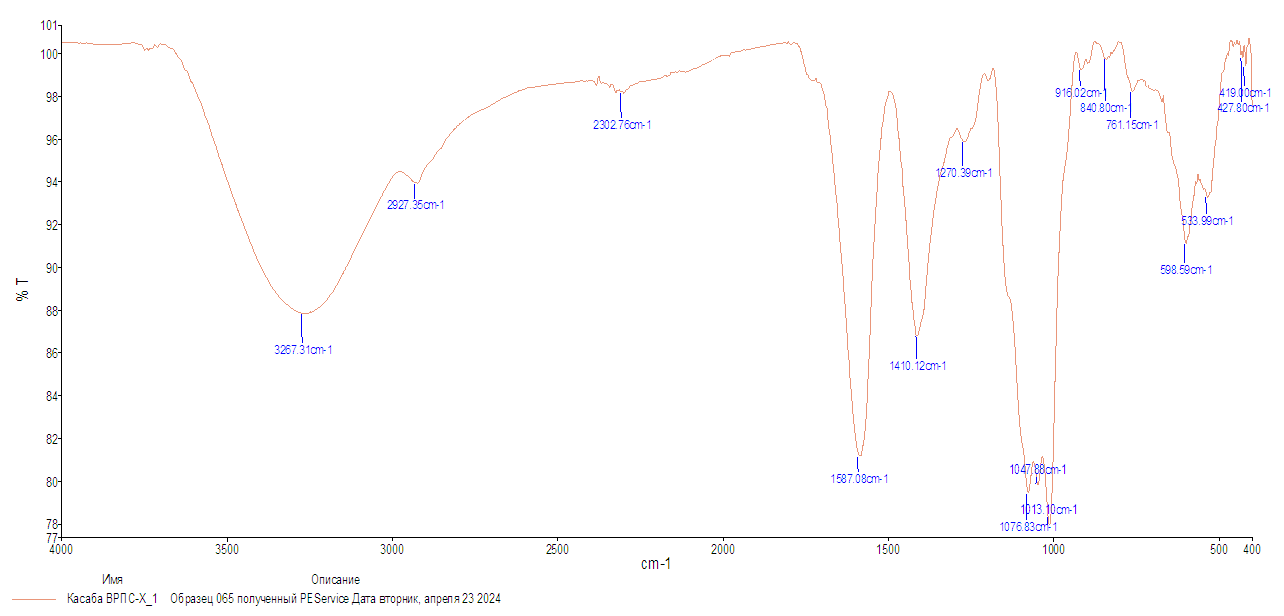 | Figure 3. IR spectrum of WSPS-c |
 | Figure 4. IR spectrum of PS |
|
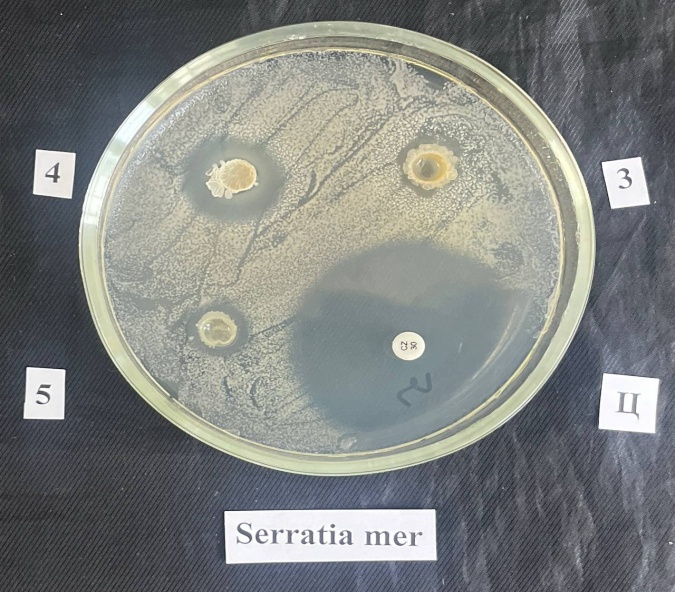 | Figure 5. Antimicrobial activity of Cucumis melo peel polysaccharides against Serratia mercescens: C. Ceftriaxone; 4. WSPS-c; 5. PS |
4. Conclusions
- Alcohol-soluble sugars, water-soluble polysaccharides, highly esterified pectin substances and hemicelluloses have been isolated from Cucumis melo peels. Their qualitative and quantitative characteristics are given. The isolated polysaccharides were analyzed by IR spectroscopy. WSPS showed an antimicrobial effect against the opportunistic pathogen Serratia marcescens.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML