-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
International Journal of Materials and Chemistry
p-ISSN: 2166-5346 e-ISSN: 2166-5354
2024; 14(4): 73-79
doi:10.5923/j.ijmc.20241404.03
Received: Aug. 16, 2024; Accepted: Sep. 10, 2024; Published: Sep. 19, 2024

Determining the Composition of Water-Soluble Polysaccharides of the Molasses of the Fruit Part of Vitis L. Plants
Azimova A. Q.1, Islamov A. X.2
1PhD Student, Faculty of Chemistry, National University of Uzbekistan, Tashkent, Uzbekistan
2DSc., Leading Researcher, Institute of Bioorganic Chemistry of the Academy of Sciences of Uzbekistan, Tashkent, Uzbekistan
Correspondence to: Azimova A. Q., PhD Student, Faculty of Chemistry, National University of Uzbekistan, Tashkent, Uzbekistan.
| Email: |  |
Copyright © 2024 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

All medicinal plants and their vegetative and generative organs are a source of metabolites for living organisms. The main purpose of the work is to study the molasses product obtained from the fruit part of mixed (Nimrang, Taipi, Rizamat, Kishmish, Saparevi) varieties of grapes in some (Kashkadarya, Navoi) regions of Uzbekistan, which is a source of carbohydrates, and its polysaccharides. Since grape molasses contains 60-70 percent carbohydrates, its separation process is difficult. Therefore, the dialysis method was used in the extraction of polysaccharides from grape molasses. The quality composition of isolated polysaccharides and quality indicators of obtained hydrolyzate and dialysate were determined by PCH method. Monosaccharide composition of isolated polysaccharide was studied by HPLCH method. According to the results, it was determined that rhamnose (7.74%), arabinose (81.0%), glucose (6.45%) and galactose (4.65%). It was found that the dominant monosaccharide is arabinose.
Keywords: Polysaccharide, Arabinose, Rhamnose, Glucose, Xylose
Cite this paper: Azimova A. Q., Islamov A. X., Determining the Composition of Water-Soluble Polysaccharides of the Molasses of the Fruit Part of Vitis L. Plants, International Journal of Materials and Chemistry, Vol. 14 No. 4, 2024, pp. 73-79. doi: 10.5923/j.ijmc.20241404.03.
1. Introduction
- The creation of clusters for cultivation, storage, primary or deep processing of medicinal plants, as well as the specialization of areas for the cultivation of medicinal plants are considered important today. As a result, the possibility of using medicinal plants and their fruit products will increase. At the same time, it causes an increase in its scope in the field of medicine and medicine. All medicinal plants and their parts (not only fruit products but also other) are a source of primary and secondary metabolites. For this purpose, the product of molasses obtained from the fruit part of various mixed vine varieties (Nimrang, Toyipi, Rizamat, Kishmish, Saparevi) considered as the object of study and its polysaccharides are considered to be of great importance. Plant carbohydrates are important biopolymers, they are part of all plants, perform various specific functions and are substances with various biological activity. Polysaccharides remove toxic substances, cholesterol, heavy metals from the body, restore damaged cells, prevent the formation of free radicals and restore the immune system. In the field of medicine, reopolyglukin, polyglukin, heparin, mukaltin and several biologically active additives have been created on the basis of polysaccharides. Many types of natural grape fruits are of particular importance due to their richness in polysaccharides. Vine is an ancient flowering or closed-seeded plant belonging to the Vitus family of the Vitaceae Juss family, which includes species close to the vine family [1]. The stem, fruit and condensed water-syrup obtained from grape species are called molasses and have been used for the treatment of various diseases since ancient times. [2]. Grape varieties are Vitis L. plant, there are a number of its species. [3]. Currently, in many countries of the world, products of the Vitis L. variety, such as syrup and vinegar, are also used for beneficial purposes. [4]. Grapes play an invaluable role in the production of biologically active and nutrient-rich foods [5]. Bekmes is a concentrate made by boiling grape molasses and grape juice for a certain time without adding sugar or other additives [6]. It is useful for diseases of the gastrointestinal tract, because almost 60-80% of bekmes consists of carbohydrates. Water, methanol and ethanol were mainly used in the research, and the antibacterial activity of different colored grape fruit extracts against infectious bacteria was studied using the method of diffusion in an agar medium [7]. Bx), biochemical properties such as pH level, titratable acidity, density, ash content, phenolic compounds and active antioxidant content were determined [8]. Bekmes is grape molasses, which is considered a high-calorie source of energy [9]. Important evidence that cyanidin-3-O-glucoside and peonidin-3-O-glucoside are the main biomolecules responsible for the color of grapes has been studied in studies [10,11]. Flavanoids with color intensity have been studied [12]. As a result, grape fruit extract had a selective effect against cancer cells, and its schematic picture was given [13]. Various cosmetic and herbal formulations are also available, including vitis oil and standardized grape seed powder [14]. The antioxidant capacity of different grape varieties, the content of chlorophylls in them and their properties have been studied [15-16]. In addition, the antioxidant and phenolic properties of grape juice and the chemical composition providing them were studied [17-18]. A total of 24 polyphenols (nine anthocyanins, three flavanols, five flavonols and seven phenolic acids) were analyzed, and the physicochemical parameters of grapes and wines were determined [19]. At the same time, the effect of grape seed extract on growth rate and immunity was analyzed in fish (common carp) [20-21]. Seedlessness of grapes, a desirable characteristic for natural consumption, confirms the use of plant plasmids as biotechnological tools [22]. The accumulation and composition changes of flavonols, proanthocyanidins and anthocyanins were measured in Vitis vinifera L. cv [23-24]. The relationship between grapevine (Vitis vinifera) vigor changes and the accumulation and composition of resulting fruit anthocyanins was investigated [25]. The relationship between grapevine (Vitis vinifera L. cv. Pinot noir) growth and the phenolic content of fruit and wine was studied [26-27]. characterized by terpenol [28-29]. individual anthocyanins in grape juices of three cultivars (Concord, Rubired and Salvador) were determined based on UV-vis and MS spectra and further determined by MS/MS spectra [30]. Systematic characterization and quantification of anthocyanins in grape juice grown in Korea [31], research on biological activity of V. vinifera in dermatological problems for cosmetic products [32]. The benefits of consuming this oil include modulation of the expression of antioxidant enzymes, anti-atherosclerotic and anti-inflammatory effects, protection against oxidative cell damage and some types of cancer [33]. Flavanoids of Cabernet Sauvignon grape varieties were compared, color intensity was studied [34]. In two varieties of Vitis vinifera L., analyzes of mineral concentration in berries of Horvatina and Barbera varieties and the number of seeds in it were carried out [35-38]. The presence of flavonoids, phenolic acids and stilbenes in red grape variety [39,41], rich sources of bioactive polyphenols [40], active components of grape extracts including grape seed, grape skin and grape juice resveratrol, phenolic acids, anthocyanins and flavonoids [41,43], anthocyanin and tannin concentration and composition of Vitis vinifera L. cv [44], grape fruit juice - Bekmes and its chemical composition, medicinal value for the body, especially in gastrointestinal activity were studied [45-47]. The scientific researches about Vitis L. plant are mainly related to dried raisin parts of stems, leaves, fruit, and the information about chemical analyzes of its fruit part condensed water-syrup (molasses) is in foreign literature, specifically O' Complete analyzes have not yet been carried out within the framework of species in the territory of Uzbekistan. Studying the carbohydrates of the fruit part of many varieties of wine grapes (Nimrang, Toyipi, Rizamat, Kishmish, Saparevi), their physico-chemical properties, monosaccharide composition and biological activity, and creating medicinal products based on them. determines the relevance of research. Polysaccharides are formed from the condensation of monosaccharides during biosynthesis. At the same time, monosaccharides are formed from the hydrolysis of polysaccharides. Polysaccharides are divided into sugar (oligosaccharides) and non-sugar types [48]. Carbohydrates become an important product in confectionery after being extracted, standardized, and in some cases modified by chemical or enzymatic processing [49]. They remove from the body microorganisms and toxins, biogenic toxins, anabolites, xenobiotics, metabolic products, as well as biologically harmful products accumulated in the body: cholesterol, bile acid, urea, bilirubin, serotonin, histamine, colon products. has the ability to be excreted from the body with itself [50].
2. Materials and Methods
- Polysaccharides are extracted from plants or other sources in various ways. The reason for this is that there is no well-defined legal rule-based method of extraction. At the same time, each time during the extraction, the additions disappear and the destruction process is different, which leads to different results. Natural polysaccharides are found in plant and animal tissues in the form of a complex system. In their extraction, 2 main requirements must be met: each process should be maximally efficient and the loss at each stage should be minimal. During the process, the polysaccharide should undergo very few changes. The extraction method is the first step in any extraction process. As a result of extraction, polysaccharides are dissolved, and additives remain undissolved. In most cases, water is used as a solvent for extraction by extraction, sometimes different solvents (DMSO DMFA and aqueous alcohol) are used to separate individual polysaccharides. With the help of such solvents, polysaccharides with a small molecular mass and not high polarity are extracted [51]. In the precipitation method, some polysaccharides dissolve and separate better in boiling water than in cold water. Therefore, they are re-precipitated using various organic solvents (methanol, ethanol, acetone, etc.) for cleaning [52]. Filtering, ultrafiltration and dialysis methods are used to clean the solution from large dispersed particles (larger than 1 μm), primary extracts of polysaccharides contain submolecular compounds: inorganic salts, proteins, etc. Ultrafiltration and dialysis are used to get rid of them. Ultrafiltration is based on passing the solution through a semi-conducting membrane (pores 0.01-1μm). Cellophane or nitrocellulose is used for ultrafiltration. Dialysis is used to separate electrolytes (sodium, sulfate and ammonium chloride ions, etc.) and micromolecular non-electrolytes (urea, sugar, alcohol, etc.) [53].Basically, high molecular compounds that cannot be separated by dialysis are purified by enzymatic method. Since grape molasses contains 60-70 percent carbohydrates, its separation process is difficult. Therefore, the dialysis method was also used in the process of extracting polysaccharides from grape molasses. 100ml (50g) of grape molasses was taken and dialyzed for 1 day due to its high molecular weight. Dialysis water was changed every 4 hours and its water was collected and poured. The obtained liquid part was pumped into the PCH(БХ) by driving it to a thick state in the rotor. The extract was taken from the dialysate and pumped in a rotor until it became thick, the volume was 40 ml. This syrup was precipitated in 1:3 acetone (120ml) (it was also precipitated in alcohol, but it did not precipitate well). The precipitate was separated by centrifugation, washed in acetone, and then dried. The mass of the resulting precipitate was 0.5 g of polysaccharide. 100mg (0.1gr) was taken from it and hydrolyzed in 1N H2SO4 at 1000C for 8 hours. The resulting hydrolyzate was neutralized with BaCO3 and deionized in cationite. Then it was filtered from the cationite and driven in a rotor until it became dry. The hydrolyzate was prepared for HPLCH(VEJX) and the sample was quantified. Quality composition of polysaccharides, obtained hydrolyzate and dialysate quality indicators were determined in PCH(БХ).
3. Result and Discussion
- In the polysaccharides determined during the experiment, PCH(БХ) contained galactose (Gal), glucose (Glc), arabinose (Ara), rhamnose (Rha) and a small amount of xylose (Xyl). In addition, the quantitative index peaks of Rhamnose (Rha), Arabinose (Ara), Xylose (Clc), Galactose (Gal) polysaccharides obtained by HPLCH(VEJX) method are shown in the following chromatogram.
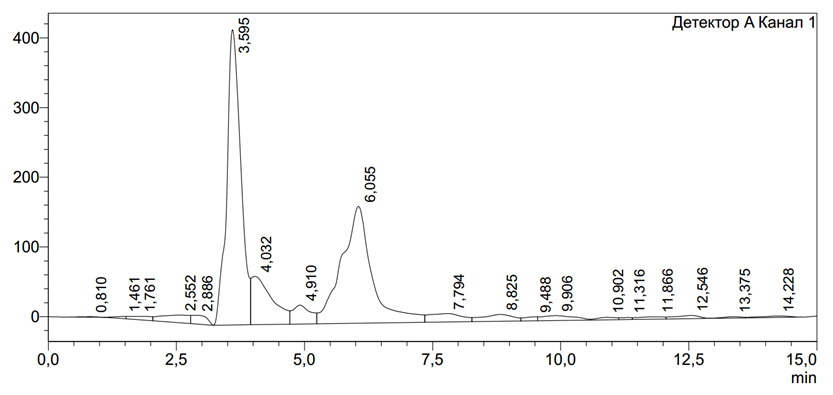 | Figure 1. Rhamnose (Rha) – 7.74%; Arabinose (Ara) – 81%; Xylose (Clc) – 6.45%; Galactose (Gal) – 4.85% |
 | Figure 2. Grape molasses polysaccharides contain galactose (Gal), glucose (Glc), arabinose (Ara), rhamnose (Rha) and a very small amount of xylose (Xyl) in PCH(БХ) |
 Due to the abundance of low molecular sugars in grape molasses, they prevent the precipitation of polysaccharides. Therefore, the dark molasses extract was slightly diluted and dialyzed for 1 day. The dialysate was centrifuged in a rotor and precipitated in 1:3 acetone. The precipitate was filtered and dried, and the polysaccharide was obtained with a yield of 2.5%. When the monosaccharide composition of the separated polysaccharide was studied by HPLCH(VEJX), it was found that rhamnose (7.74%), arabinose (81.0%), glucose (6.45%) and galactose (4.65%). It was found that the dominant monosaccharide is arabinose.
Due to the abundance of low molecular sugars in grape molasses, they prevent the precipitation of polysaccharides. Therefore, the dark molasses extract was slightly diluted and dialyzed for 1 day. The dialysate was centrifuged in a rotor and precipitated in 1:3 acetone. The precipitate was filtered and dried, and the polysaccharide was obtained with a yield of 2.5%. When the monosaccharide composition of the separated polysaccharide was studied by HPLCH(VEJX), it was found that rhamnose (7.74%), arabinose (81.0%), glucose (6.45%) and galactose (4.65%). It was found that the dominant monosaccharide is arabinose.4. Conclusions
- It was concluded that grape molasses contains few high molecular sugars and high content of low molecular sugars glucose, fructose and sucrose.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML