-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
International Journal of Materials and Chemistry
p-ISSN: 2166-5346 e-ISSN: 2166-5354
2024; 14(4): 67-72
doi:10.5923/j.ijmc.20241404.02
Received: Jul. 19, 2024; Accepted: Aug. 10, 2024; Published: Aug. 30, 2024

Synthesis and Investigation of Amino-carboxy-sulfonate Amphoteric Ionite Obtained by Methods of Transformation and Modification of Polymers
Zakirkhodja A. Tadjihodjaev1, Minira R. Sodikova2
1Tashkent Institute of Chemical Technology, Tashkent, Uzbekistan
2Tashkent Scientific Research Institute of Chemical Technology, Tashkent Region, Uzbekistan
Correspondence to: Minira R. Sodikova, Tashkent Scientific Research Institute of Chemical Technology, Tashkent Region, Uzbekistan.
| Email: |  |
Copyright © 2024 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

A comprehensive approach has been developed to solve the problem of using a secondary product of fat-and-oil production and secondary polymer materials as resource-saving raw materials in the creation of new functional ion-exchange materials. Alternative technological solutions for the trasformation and modification of the developed ion–exchange materials are proposed in order to obtain amino–carboxy-sulfonate ionite sorbent by introducing additional strong acid sulfogroups or by introducing spent (in water treatment processes) cationite into the matrix as a functional component. The modified amphoteric ionite, which has a functional component in its composition – spent «Kу-2-8» cationite, was studied by scanning electron microscopy (SEM) in order to study the structure of the nanostructure and microstructure of the surface and inner region of ion-exchange materials. Amphoteric ionite, which has a functional component in its composition, has predominantly (≤ 26%) the best exchange capacity values for 0.1 n. NaOH solution is 5.2–5.4 mg-eq/g in comparison with an unmodified ion-exchange material.
Keywords: Ionite, Sulfonation, Modification, Scanning electron microscope, «Kу-2-8», Ion exchange matrices, Polyethylenepolyamine, Furfural, Oleum, Olein-palmitic fraction of cotton soapstock
Cite this paper: Zakirkhodja A. Tadjihodjaev, Minira R. Sodikova, Synthesis and Investigation of Amino-carboxy-sulfonate Amphoteric Ionite Obtained by Methods of Transformation and Modification of Polymers, International Journal of Materials and Chemistry, Vol. 14 No. 4, 2024, pp. 67-72. doi: 10.5923/j.ijmc.20241404.02.
Article Outline
1. Introduction
- A rational approach to the treatment of secondary products and industrial waste, as well as consumer products, is to maximize their processing into other products with increased added value.The analysis of scientific and technical literature on waste from the fat and oil industry and secondary polymers, namely secondary polyethylene terephthalate, predetermined the development of a new technological direction for the integrated use of secondary raw materials of cottonseed oil, namely olein-palmitic fraction/mixture and chemically processed secondary polyethylene terephthalate as the most acceptable secondary products for use in the production of ion exchange materials and inhibitory compositions.The choice of these secondary products is justified by the fact that a huge amount of plastic waste, including in the form of recycled polyethylene terephthalate (PET containers), partially lost their properties, but retained their technological properties, make it possible to recycle them by chemical modification for reuse as feedstock to obtain new types of chemical products – ion exchange materials and inhibitory compositions.Considering that PET containers decompose in natural conditions for centuries, during this time solid waste will accumulate in huge quantities, polluting and clogging the planet.Another class of unrealized polymers and processing technology for which has not been developed are secondary products of mesh (crosslinked) polymers, namely spent synthetic ionites. Used synthetic ionites have been unclaimed to date and have not been considered as secondary raw materials. However, such properties of spent ionites as a sufficiently high residual sorption capacity, the identity of the chemical composition of newly developed ion exchange materials indicate the prospects of their use as a functional component in the production of various types of ionites and sorbents.In the production of cottonseed oil and fatty acids, depending on the technological scheme and methods of separating the main products, many secondary products and waste are formed, the processing of which plays an important role. In our research, the olein-palmitic fraction/mixture of cotton soapstock will be investigated and used.The aim of the research is to provide alternative technological solutions for the modification of ion-exchange materials in order to obtain an amino-carboxy-sulfonate polymer by methods of transformation of strongly acidic sulfogroups and modification by introducing regenerated spent strongly acidic cationite «Kу-2-8» into the matrix as a functional component.The research is aimed at:Firstly, the secondary use of polymer waste and secondary products of fat-and-oil production in order to obtain new types of ion-exchange materials, which is important from an environmental point of view and from the perspective of rational use of raw materials.Secondly, it is aimed at a comprehensive solution of technological issues aimed at the development and creation of polymer – polymer ion-exchange materials for multifunctional purposes with high functional and operational characteristics of application.Comprehensive studies of the regularities of the synthesis of ion–exchange matrices in the presence of spent ionites as polymer-dispersed nanomaterials have been carried out, the features of their structure formation and the analysis of the interrelationships of the structure and properties of ion-exchange materials have been revealed. For the first time, the technological foundations for the production of filled ion-exchange materials for multi-purpose purposes are formulated. Since the use of spent ionites depends on the dispersion, the required degree of dispersion was determined taking into account the direction of ionite use.When synthesizing ion-exchange matrices, the complex structure of ion-exchange materials should be taken into account, including gel sections in which the polymer itself and spent ionites represent separate phases. On this basis, the gaps in the gel regions are classified as micro- and mesopores, where functional groups of spent ionites are also localized on the walls of these pores.The increasing consumption of ionites by various industries requires the development and improvement of methods for obtaining alternative ion exchange materials through previously unknown or little-known technological solutions for the creation of promising new generation ionites.The structures of nanostructures and microstructures of ion-exchange materials have been studied, as well as their physico-mechanical and technological properties have been studied. Currently, electron microscopy is the main direct method of studying the structure of nanostructures and microstructure of ion-exchange materials. Its main advantages over other microscopy methods are: direct instantaneous image formation, a wide range of easily variable magnifications, a large depth of focus at high resolution, the possibility of diffraction examination (and, consequently, obtaining a variety of information about the internal structure of an ion-exchange material and its state: structure, ordering, porosity, etc.), the possibility of microrentgenospectral (elemental) analysis, etc.The research has undertaken a theoretical study of the mechanism of the chemical reaction of an «АНФ» by polycondensation of styrene, furfural in the presence of polyethylenepolyamine (PEPA) and its subsequent sulfonation [1-4].Alternative technological solutions for the creation of ion-exchange materials are proposed, which are considered in two directions: first, the introduction of additional strongly acidic sulfogroups (–SO3H) to amphoteric ionites, which already have weakly acidic carboxyl groups (–COOH) and weakly basic tertiary and secondary amino groups (–N+R2H and –N+RH2) in their structure [5] in order to obtain amino-carboxy-sulfate amphoteric ionite with improved exchange capacity by cationite; secondly, modification of the developed ion-exchange materials by introducing regenerated spent synthetic ion-exchange materials of any type into the matrix, depending on their purpose and application.Technological solutions for the synthesis of amino-carboxy-sulfonate ion exchange material obtained by methods of transformation of sulfo groups and modification of polymers by introducing a functional component up to 20 wt.% make it possible to obtain new ion-exchange materials with amphoteric properties and improved exchange capacity values for cationic groups.In connection with the above, we have previously obtained a number of ion-exchange materials and studied their basic physico-chemical and sorption properties [6-9].
2. Main Body
2.1. Explanation of Methods
- Amino-carboxy-sulfonate amphoteric ionite AR&ISM-12 was obtained by the transformation method. At the first stage, polycondensation of the olein-palmitic fraction/mixture of cotton soapstock [10] with a secondary product of polymer products (SPPP), PEPA and furfural produced amphoteric ionite containing carboxylic, secondary and tertiary amino groups. Amphoteric ionites with sufficiently good properties were obtained at a mass ratio of olein palmitic fraction of cotton soapstock (OPF CS) to SPPP, PEPA and furfural 0.5:0.5:1.0:1.0 and a temperature of 90-110°C, conventionally designated AR&ISM-10 with exchange capacities EC, mg-equiv/g of 0.1 n. NaOH solution - 4.0; 0.1 n. HCl solution – 4.9.Similarly, the polymer matrix AR&ISM-11 was obtained by chemical interaction of OPF CS with SPPP and furfural 0.5:0.5:1.0, that is, on the basis of a three-component system acceptable for functionalization by sulfonic acid groups.At the second stage, sulfonation of a three-component polymer matrix AR&SM-11 pre-swollen in a sulfonating agent was carried out. To carry out sulfonation of AR&ISM-11, 70-98% sulfuric acid, oleum with different SO3 content were used. The mass ratio of the sulfonating agent to ionite was 5:1; the sulfonation time was 4-8 hours, the temperature was 70-110°C. The resulting amino-carboxy-sulfonate amphoteric ionite AR&ISM-12 was purified from unreacted starting materials by sequential washing with 5% solutions of HC1→H2O distilled water, NaOH→H2O distilled water to a neutral reaction of washing waters and polymer granules were dried to an air-dry state.At the third stage, the modification of the developed ion exchange materials AR&ISM-10 was carried out by introducing the purified strongly acidic cationite «Kу-2-8» (cationite used in water treatment processes) into the matrix as a functional component in order to obtain modified amphoteric ionite AR&ISM-13.Amphoteric ionites with sufficiently good properties were obtained at a mass ratio of OPF CS: «Kу-2-8» to SPPP, PEPA and furfural 0.5:0.5:0.5:1.0:1.0 and a temperature of 80-90°C, conventionally designated AR&ISM-13 with an exchange capacity of 0.1 n. NaOH solution (cationic groups), which have indicators 5.2-5.4 mg-eq/g, of which for carboxyl groups (SECCOOH) 3.1-3.2 mg-eq/g; for sulfogroups (SECSO3H) 2.1-2.2 mg-eq/g.
2.2. Results and Discussion
- Considering that during sulfonation, the reaction rate decreases as a result of dilution of sulfuric acid with water formed by the reaction, and the substitution reaction eventually stops, an excess of sulfuric acid or oleum was used in studies to shift the sulfonation equilibrium to the right side.Sulfonation of the cured polymer matrix (resin) AR&ISM-11 with sulfuric acid (d=1.84 at 5 times the weight excess) for 10 hours produced ionite with an exchange capacity of 0.1 n. NaOH solution is 0.5-0.7 mg-equiv/g higher than the exchange capacity of AR&ISM-10. The next stage of the experiments should be carried out using 5% oleum as a sulfonating agent. The resulting amino-carboxy-sulfonate amphoteric ionite, conventionally designated as – AR&ISM-12, has an exchange capacity of 0.1 n. NaOH solution – 5.1-5.2 mg-eq/g. The data characterizing the dependence of the exchange capacity on the sulfonation conditions at a weight ratio of 5% oleum: AR&ISM-11 – 5:1 is shown in Figure 1.
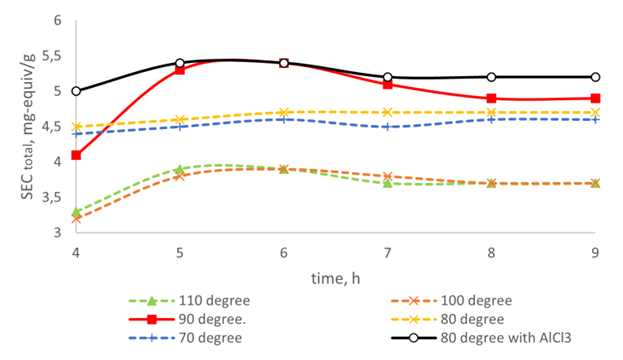 | Figure 1. Dependence of the exchange capacity on the temperature and time of sulfonation |
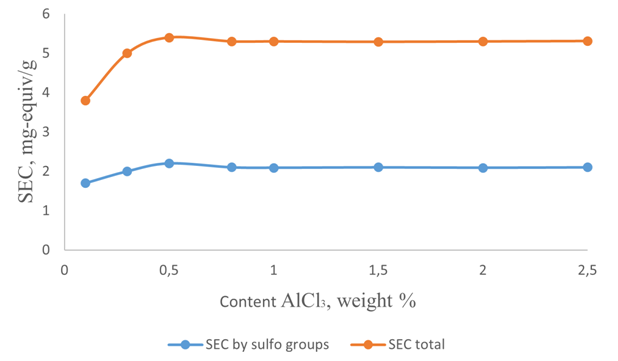 | Figure 2. Dependence of the exchange capacity on the amount of catalyst during sulfonation for 4 hours at 80°C |
 | Figure 3. Electron microscopic image of the ionite structure (magnification x230) |
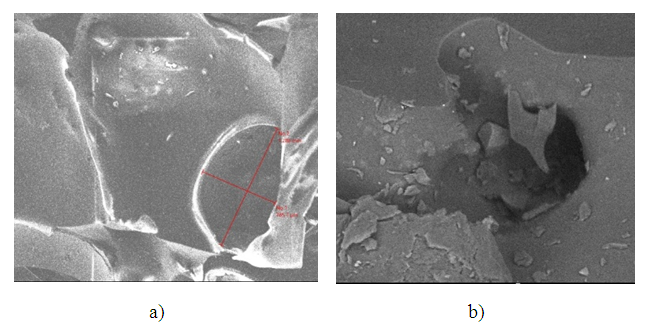 | Figure 4. Electron microscopic image of the ionite surface structure (x230) |
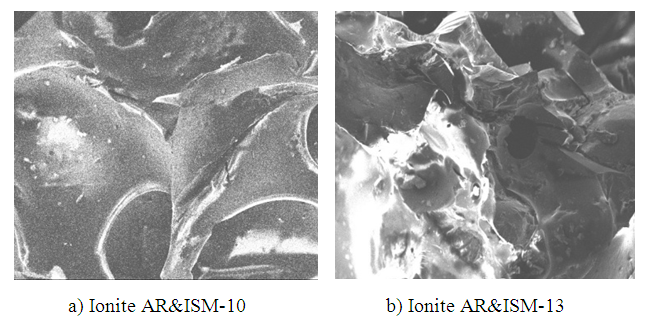 | Figure 5. Mapping of synthesized ionites after repeated regeneration (x230) |
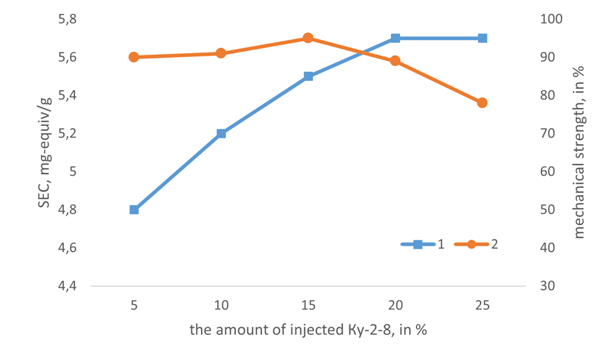 | Figure 6. Dependences of EC (1) and mechanical strength (2) of AR&ISM13 on the amount of the introduced modifying reagent |
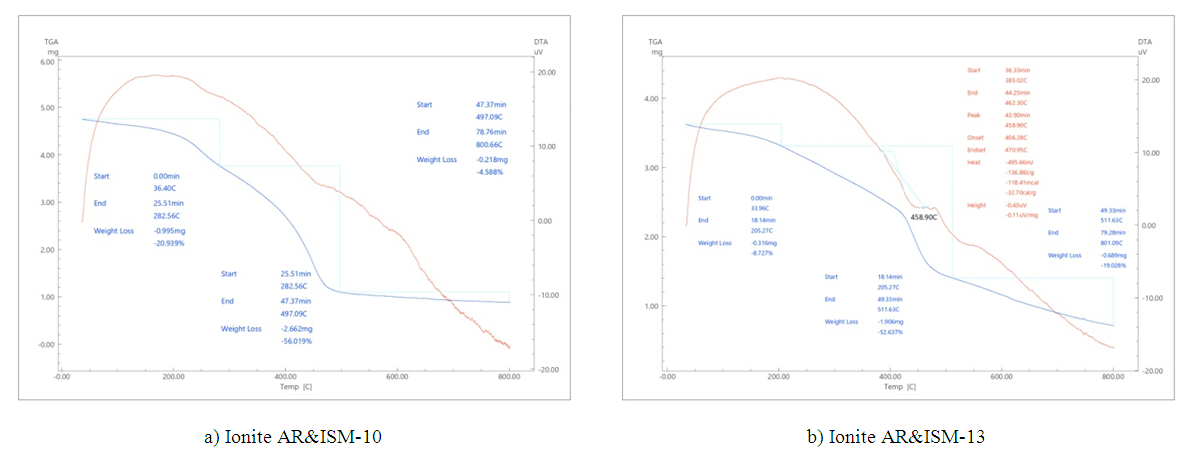 | Figure 7. TGA and DTA of ion exchange materials |
|
3. Conclusions
- • By chemical interaction of the olein-palmitic fraction /mixture of cotton soapstock with SPPP and furfural 0.5:0.5:1.0, that is, on the basis of a three-component system, a polymer matrix acceptable for functionalization by sulfonic acid groups was obtained. The resulting amino-carboxy-sulfonate amphoteric ionite has an exchange capacity of 0.1 n. NaOH solution – 5.1-5.2 mg-eq/g. • Modification of previously developed ion-exchange materials obtained by polycondensation of olein-palmitic fraction/ mixture of cotton soapstock with SPPP, PEPA and furfural was carried out by introducing into the matrix the regenerated strongly acidic cationite «Kу-2-8» (cationite used in water treatment processes) as a functional component in order to obtain a modified amphoteric ionite with an exchange capacity of 0.1 N. NaOH solution – 5.2-5.4 mg-eq/g.• The developed ionite compositions using spent ionites make it possible to reduce the cost of production and obtain ion-exchange materials with the required operational properties (good mechanical strength, thermal stability and exchange capacity).
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML