| [1] | Gökhan Gece, 2008 The use of quantum chemical methods in corrosion inhibitor studies, Corrosion Science, 50, (11), 2981-2992. |
| [2] | Yadav M., Behera D., Kumar S., Sinha R.,R., 2013, Experimental and quantum chemical studies on the corrosion inhibition performance of benzimidazole derivatives for mild steel in HCl. Industrial & Engineering Chemistry Research, 52, 6318-28. |
| [3] | Qiang Y., Zhang S., Tan B., Chen S., 2018, Evaluation of Ginkgo leaf extract as an eco-friendly corrosion inhibitor of X70 steel in HCl solution. Corrosion Science 133, 6–16. |
| [4] | Finšgar M., Jackson J., 2014, Application of corrosion inhibitors for steels in acidic media for the oil and gas industry: a review. Corrosion Science 86, 17-41. |
| [5] | Assad H., Kumar, A., 2021, Understanding functional group effect on corrosion inhibition efficiency of selected organic compounds. Journal of Molecular Liquids 344, 117755. |
| [6] | Tang Y., Yang X., Yang W., Chen Y., Wan R., 2010, Experimental and molecular dynamics studies on corrosion inhibition of mild steel by 2-amino-5-phenyl-1,3,4- thiadiazole. Corros Science 52, 242–9. |
| [7] | Fouda, A. S., Ismail, M. A., Abousalem, A. S., & Elewady, G. Y., 2017, Experimental and theoretical studies on corrosion inhibition of 4-amidinophenyl-2, 2′-bifuran and its analogues in acidic media. RSC advances, 7 (73), 46414-46430. |
| [8] | Peter, A., Obot, I. B., & Sharma, S. K., 2015, Use of natural gums as green corrosion inhibitors: an overview. International Journal of Industrial Chemistry 6, 153-164. |
| [9] | Becke, D., 1986, Density functional calculations of molecular bond energies. The Journal of Chemical Physics 84, 4524-4529. |
| [10] | Lee, C., Yang, W., and Parr, R. G., 1988, Development of the colle-salvetti correlation-energy formula into a functional of the electron density. Physical Review B., 37, 785-789. |
| [11] | M. J. Frisch, G. W. Trucks, H. B. Schlegel, G. E. Scuseria, M. A. Robb, J. R. Cheeseman, G. Scalmani, V. Barone, B. Mennucci, G. A. Petersson, H. Nakatsuji, M. Caricato, X. Li, H. P. Hratchian, A. F. Izmaylov, J. Bloino, G. Zheng, J. L. Sonnenberg, M. Hada, M. Ehara, K. Toyota, R. Fukuda, J. Hasegawa, M. Ishida, T. Nakajima, Y. Honda, O. Kitao, H. Nakai, T. Vreven, J. A. Montgomery, Jr., J. E. Peralta, F. Ogliaro, M. Bearpark, J. J. Heyd, E. Brothers, K. N. Kudin, V. N. Staroverov, R. Kobayashi, J. Normand, K. Raghavachari, A. Rendell, J. C. Burant, S. S. Iyengar, J. Tomasi, M. Cossi, N. Rega, J. M. Millam, M. Klene, J. E. Knox, J. B. Cross, V. Bakken, C. Adamo, J. Jaramillo, R. Gomperts, R. E. Stratmann, O. Yazyev, A. J. Austin, R. Cammi, C. Pomelli, J. W. Ochterski, R. L. Martin, K. Morokuma, V. G. Zakrzewski, G. A. Voth, P. Salvador, J. J. Dannenberg, S. Dapprich, A. D. Daniels, Ö. Farkas, J. B. Foresman, J. V. Ortiz, J. Cioslowski and A. D. J. Fox, Gaussian, Inc., Wallingford, (2009), 09. |
| [12] | Koopmans T., 1933, Ordering of wave functions and eigen energies to the individual electrons of an atom, Physica, 1, 104–113. |
| [13] | Allal, H., Belhocine, Y., and Zouaoui, E., 2018, Computational study of some thiophene derivatives as aluminium corrosion inhibitors. Journal of Molecular Liquids 265, 668–678. |
| [14] | R. G. Parr and R. G. Pearson, 1983, Absolute hardness: companion parameter to absolute electronegativity. Journal of the American Chemical Society, 105, 7512-7516. |
| [15] | R. G. Parr, L. Szentpaly and S. Liu, 1999, Electrophilicity index. Journal of the American Chemical Society, 121, 1922-1924. |
| [16] | Pearson, R. G., 1988, Absolute electronegativity and hardness: application to inorganic chemistry. Inorganic Chemistry, 27 (4), 734-740. |
| [17] | Reed, A. E., Curtiss, L. A., & Weinhold, F., 1988, Intermolecular interactions from a natural bond orbital, donor-acceptor viewpoint. Chemical Reviews, 88(6), 899-926. |
| [18] | K. Fukui, T. Yonezawa, H. Shingu, 1952, The Journal of Chemical Physics, 4, 722-725. |
| [19] | Yang, W., Mortier, W. J., 1986, The use of global and local molecular parameters for the analysis of the gas-phase basicity of amines, Journal of American Chemistry Society, 108 (19), 5708-5711. |
| [20] | Christophe Morell André Grand Alejandro Toro-Labbé, 2004, New Dual Descriptor for Chemical Reactivity, Journal of Physical Chemistry, A, 109(1): 205-212. |
| [21] | Christophe Morell, André Grand, Alejandro Toro-Labbé, 2006, Theoretical support for using the Δf(r) descriptor, Chemical Physics Letters, Volume 425, Issues 4–6, Pages 342-346. |
| [22] | K. Fukui, 1982, The role of frontier orbitals in chemical reactions (Nobel Lecture). Angewandte Chemie International Edition in English, 21(11) 801-809. |
| [23] | Z. Zhang, W. Li, W. Zhang, X. Huang, L. Ruan, L. Wu, 2018, Experimental, quantum chemical calculations and molecular dynamics (MD) simulation studies of methionine and valine as corrosion inhibitors on carbon steel in phase change materials (PCMs) solution. Journal of Molecular Liquids, 272 528-538. |
| [24] | R. Nabah, F. Benhiba, Y. Ramli, M. Ouakki, M. Cherkaoui, H. Oudda, A. Zarrouk, 2018 Corrosion Inhibition Study of 5, 5-diphenylimidazolidine-2, 4-dione for Mild Steel Corrosion in 1 M HCl Solution: Experimental, Theoretical Computational and Monte Carlo Simulations Studies. Analytical & Bioanalytical Electrochemistry,10-10 1375-1398. |
| [25] | Kaya S., Banerjee P., Saha S. K., Tüzün B., Kaya C.,2016, Theoretical evaluation of some benzotriazole and phospono derivatives as aluminum corrosion inhibitors: DFT and molecular dynamics simulation approaches, RSC Advances, 6,74550-74559. |
| [26] | Kaya S., Tüzün B., Kaya C., Obot I.B., 2016, Determination of corrosion inhibition effects of amino acids: Quantum chemical and molecular dynamic simulation study, Journal of the Taiwan Institute of Chemical Engineers, 58, 528-553. |
| [27] | Kaya S., Kaya C., Guo L., Kandemirli F., Tüzün B., Uğurlu İ., Madkour L.H., Saraçoğlu M., 2016, Quantum chemical and molecular dynamics simulation studies on inhibition performances of some thiazole and thiadiazole derivatives against corrosion of iron. Journal of Molecular Liquids, 219 497-504. |
| [28] | Ibeji, C.U., Adejoro, I.A. and Adeleke, B.B., 2015, A benchmark study on the properties of unsubstituted and some substituted polypyrroles Journal of Phys Chem Biophys,. 5: p. 1. |
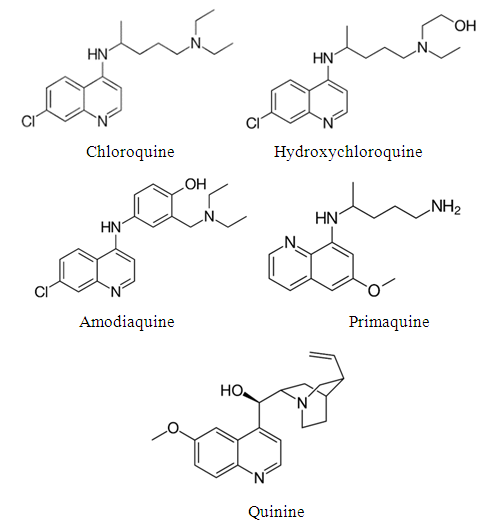
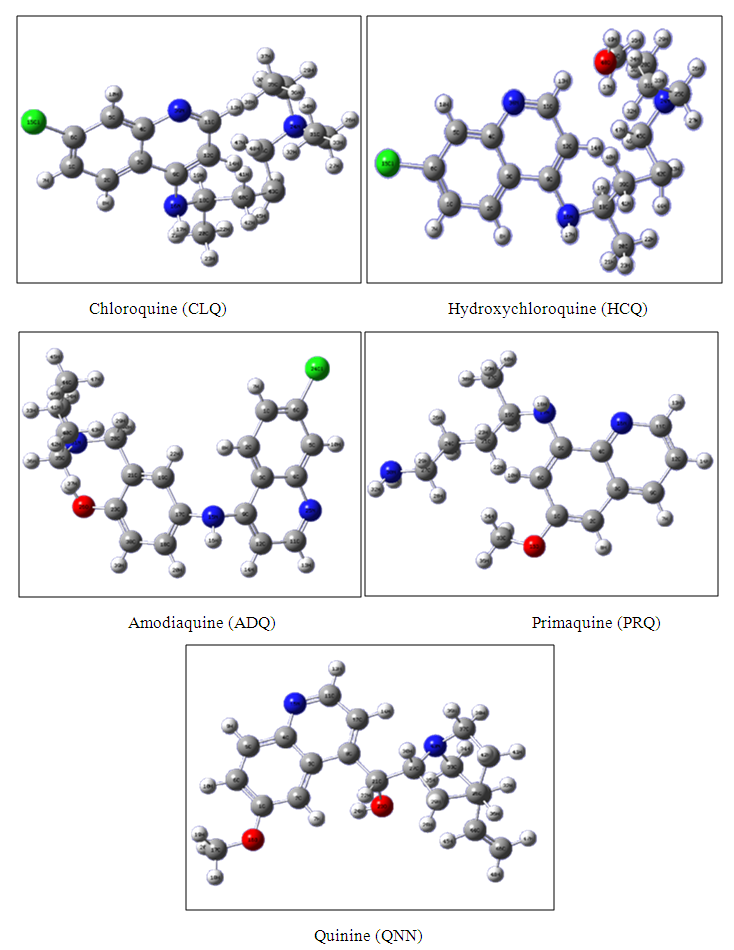
| [29] | Adejoro, I. A., Akintayo, D. C., & Ibeji, C. U., 2016, The efficiency of chloroquine as corrosion inhibitor for aluminium in 1M HCl solution: Experimental and DFT study. Jordan Journal of Chemistry 11 (1), 38-49. |
| [30] | Gece, G., Bilgiç, S. 2009. Quantum chemical study of some cyclic nitrogen compounds as corrosion inhibitors of steel in NaCl media. Corrosion Science, 51(8), 1876–1878. |
| [31] | Kikuchi, O., 1987, Systematic QSAR procedures with quantum chemical descriptors. Quantitative Structure- Activity Relationships, 6 (4), 179-184. |
| [32] | V.G. Vasudha, K.S. Priya, Chem. Sci. Rev. Lett., 2014, 2, 435–443. |
| [33] | Ebenso, E. E., Isabirye, D. A., Eddy, N. O., 2010, Adsorption and quantum chemical studies on the inhibition potentials of some thiosemicarbazides for the corrosion of mild steel in acidic medium. International journal of molecular sciences, 11(6), 2473-2498. |
| [34] | M. Touil, N. Hajjaji, D. Sundholm, H., Rabaâ, 2013 International Journal of Quantum Chemistry, 113 1365. |
| [35] | Fukui, K., Yonezawa, T., & Shingu, H. 1952, A molecular orbital theory of reactivity in aromatic hydrocarbons. The Journal of Chemical Physics, 20 (4), 722-725. |
| [36] | Yadav M, Sharma D, Sarkar T. K., 2015, Adsorption and corrosioninhibitive properties of synthesized hydrazine compounds on N80steel/hydrochloric acid interface: electrochemical and DFT studies.J Mol Liq 212: 451–460. |
| [37] | Lukovits, I., Kalman, E., & Zucchi, F., 2001, Corrosion inhibitors-correlation between electronic structure and efficiency. Corrosion, 57(1), 3-8. |
| [38] | Christophe Morell André Grand Alejandro Toro-Labbé, 2004, New Dual Descriptor for Chemical Reactivity, Journal of Physical Chemistry, A, 109 (1): 205-212. |
| [39] | Martínez-Araya, 2015, J.I. Why is the dual descriptor a more accurate local reactivity descriptor than Fukui fun ctions? J Math Chem., 53. 451–465. |



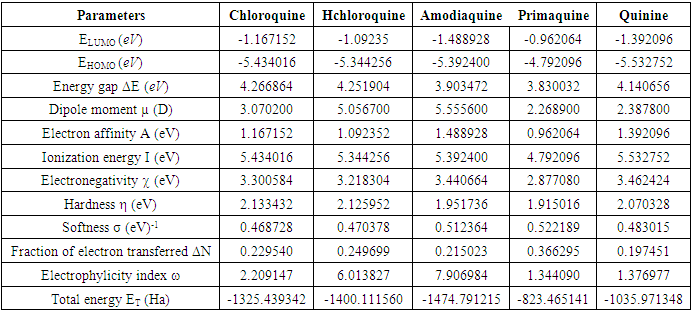
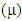 , total energy (TE), electronegativity
, total energy (TE), electronegativity 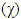 , hardness
, hardness 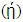 , the number of transferred electrons (ΔN), electron affinity
, the number of transferred electrons (ΔN), electron affinity 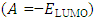 [12], ionization energy
[12], ionization energy 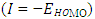 [12] etc. are identified. Some parameters such electronegativity
[12] etc. are identified. Some parameters such electronegativity 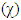 , hardness
, hardness 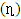 , softness
, softness 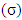 , affinity and electrophylicity index
, affinity and electrophylicity index 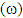 are related to ionization energy
are related to ionization energy 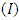 and electron affinity
and electron affinity 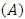 . They are expressed from the following relationships [13-15]:
. They are expressed from the following relationships [13-15]: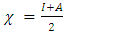
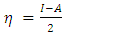
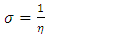
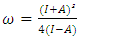
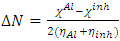
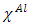 and
and 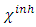 represent the absolute electronegativity of Al and the inhibitor molecule, respectively,
represent the absolute electronegativity of Al and the inhibitor molecule, respectively, 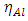 and
and 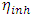 represent the absolute hardness of Al and the inhibitor molecule, respectively. By assuming that for a metallic bulk
represent the absolute hardness of Al and the inhibitor molecule, respectively. By assuming that for a metallic bulk 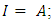 because they are softer than the neutral metallic atoms [18]; theoretical values for the electronegativity
because they are softer than the neutral metallic atoms [18]; theoretical values for the electronegativity 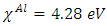 and
and 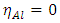 the global hardness were hence used.Net atomic charges have been obtained using the Natural Population Analysis (NPA) of Weinhold [19]. The population analysis has been performed on the neutral, cationic and anionic species at the same obtained optimized geometry of each inhibitor in order to determine their Fukui functions. Molecules local selectivity parameters such as Fukui functions and dual descriptor responsible for nucleophilic and electrophilic attacks was also determined [20-24]. The following equations were used to determine these parameters [25]:
the global hardness were hence used.Net atomic charges have been obtained using the Natural Population Analysis (NPA) of Weinhold [19]. The population analysis has been performed on the neutral, cationic and anionic species at the same obtained optimized geometry of each inhibitor in order to determine their Fukui functions. Molecules local selectivity parameters such as Fukui functions and dual descriptor responsible for nucleophilic and electrophilic attacks was also determined [20-24]. The following equations were used to determine these parameters [25]: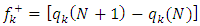
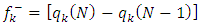
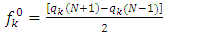
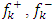 and
and 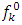 are respectively nucleophilic, electrophilic and radical Fukui functions, (𝑁+1), (𝑁) and (𝑁−1) are the electronic population of atom 𝑘 in (𝑁+1), 𝑁 and (𝑁−1) electrons systems. The condensed form of dual descriptor is given in equation (13)
are respectively nucleophilic, electrophilic and radical Fukui functions, (𝑁+1), (𝑁) and (𝑁−1) are the electronic population of atom 𝑘 in (𝑁+1), 𝑁 and (𝑁−1) electrons systems. The condensed form of dual descriptor is given in equation (13)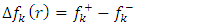
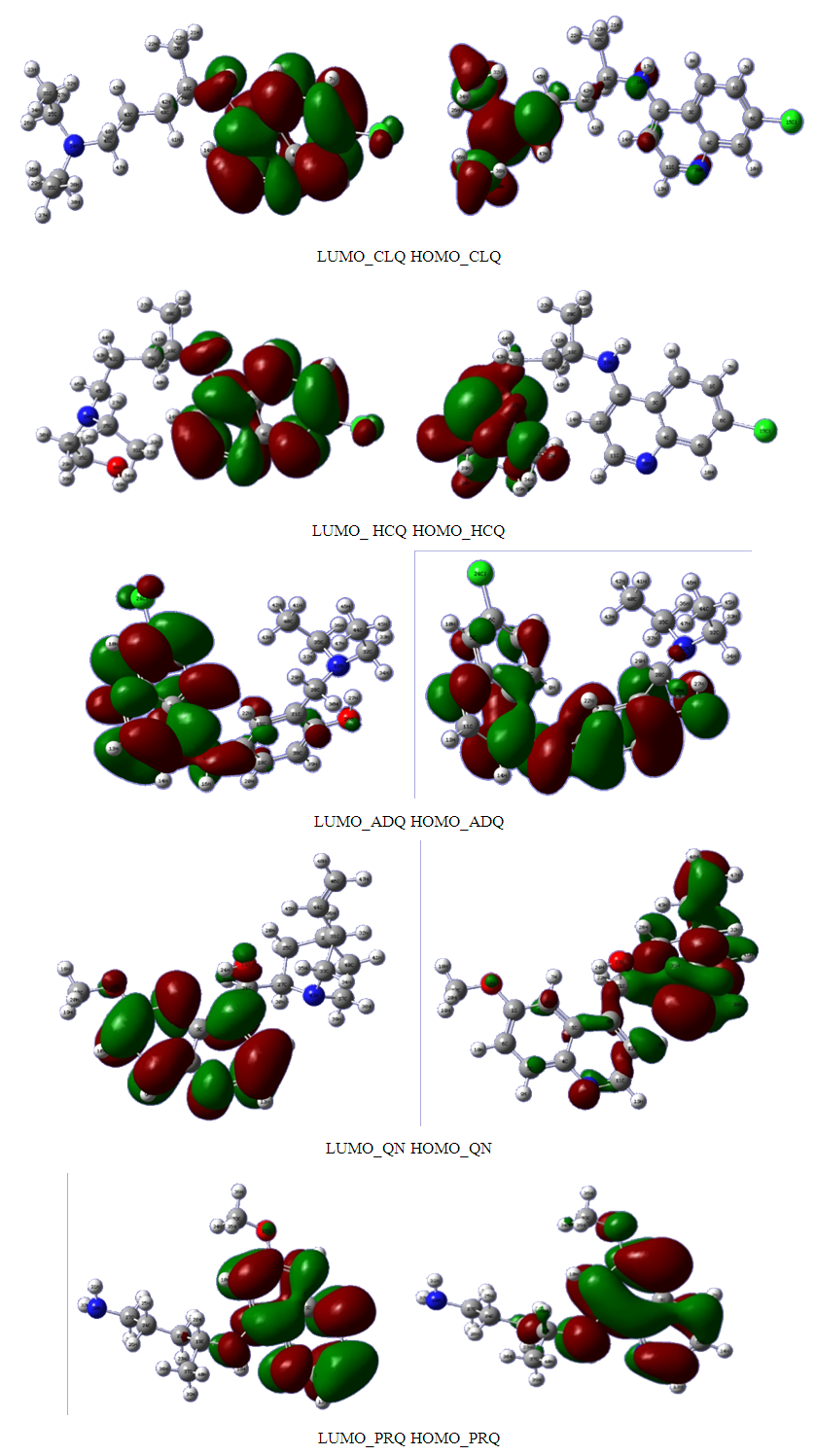
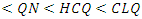 in B3LYP method. Therefore, their higher reactivity can allowed them to be easily adsorbed onto the metal (Al) surface leading to increase their inhibitive efficiencies compared respectively. The results also show that ADQ and QNN have the lowest
in B3LYP method. Therefore, their higher reactivity can allowed them to be easily adsorbed onto the metal (Al) surface leading to increase their inhibitive efficiencies compared respectively. The results also show that ADQ and QNN have the lowest 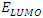 compared to others molecules respectively which indicate a better capability of ADQ and QNN to accept electrons and since a good corrosion inhibitor should not only be a good electrons donor but also a good electron acceptor through the back donation mechanism. This result can lead to increase their adsorption on the metal surface and accordingly increases their inhibition efficiency. Absolute hardness and softness are very important parameters to describe the molecular reactivity and stability. The absolute hardness η exemplifies the resistance of a molecule to electron charge transfer. Given that the corrosion inhibition process always involves the transfer of electrons between the inhibitor and the metallic surface. Thereby, soft molecules of low hardness are more responsive to charge transfer. Soft molecules are more reactive than hard ones because they can easily offer electrons. Hence, inhibitors with the highest values of global softness (the least values of the global hardness) are expected to be good corrosion inhibitors for bulk metals in acidic media.The calculations from table 1 indicate that PRQ (0.522189) and ADQ (0.512364) have the highest softness (σ) values compared to QNN (0.483015), HCQ (0.470378) and CLQ (0.468728), respectively. This result was consistent with the general belief that hard molecules should have large ∆E and a soft molecule should have small ∆E [32].As for the dipole moment, there is an inconsistent in the literature on the correlation between the dipole moment and inhibition efficiency [33,34]. However, it is generally agreed that the adsorption of compounds possessing high dipole moments on the metal surface should lead to better inhibition efficiency [35]. Indeed, the results indicate that ADQ and HCQ have the highest values of μ compared to CLQ, QNN and PRQ, respectively. The fraction of electrons transferred describes the trend of electrons donation within a set of inhibitors. Indeed, the fraction of electron transferred ΔN presents the particularity of considering both the metal and the inhibitor (in terms of χ and η) to determine the direction and intensity of the electrons’ flow. The positive values of ΔN for both inhibitors indicate that the flow of electrons takes place probably from the inhibitors towards the metal. Also, it is generally assumed that if ΔN<3.6 the inhibition efficiency is found to amplify as the values of ΔN increase [36].According to Lukovits’s study [37], if ΔN < 3.6 then the inhibition efficiency increased with increasing electron-donating ability at the metal surface. The obtained values of ΔN reported in Table 1 are all below 3.6 and PRQ and HCQ have higher values of ΔN than ADQ, CLQ and QNN respectively. This result implies good disposition of PRQ and HCQ molecules to donate their electrons leading to increase their adsorption on the metal surface and to increase their inhibition efficiencies.
compared to others molecules respectively which indicate a better capability of ADQ and QNN to accept electrons and since a good corrosion inhibitor should not only be a good electrons donor but also a good electron acceptor through the back donation mechanism. This result can lead to increase their adsorption on the metal surface and accordingly increases their inhibition efficiency. Absolute hardness and softness are very important parameters to describe the molecular reactivity and stability. The absolute hardness η exemplifies the resistance of a molecule to electron charge transfer. Given that the corrosion inhibition process always involves the transfer of electrons between the inhibitor and the metallic surface. Thereby, soft molecules of low hardness are more responsive to charge transfer. Soft molecules are more reactive than hard ones because they can easily offer electrons. Hence, inhibitors with the highest values of global softness (the least values of the global hardness) are expected to be good corrosion inhibitors for bulk metals in acidic media.The calculations from table 1 indicate that PRQ (0.522189) and ADQ (0.512364) have the highest softness (σ) values compared to QNN (0.483015), HCQ (0.470378) and CLQ (0.468728), respectively. This result was consistent with the general belief that hard molecules should have large ∆E and a soft molecule should have small ∆E [32].As for the dipole moment, there is an inconsistent in the literature on the correlation between the dipole moment and inhibition efficiency [33,34]. However, it is generally agreed that the adsorption of compounds possessing high dipole moments on the metal surface should lead to better inhibition efficiency [35]. Indeed, the results indicate that ADQ and HCQ have the highest values of μ compared to CLQ, QNN and PRQ, respectively. The fraction of electrons transferred describes the trend of electrons donation within a set of inhibitors. Indeed, the fraction of electron transferred ΔN presents the particularity of considering both the metal and the inhibitor (in terms of χ and η) to determine the direction and intensity of the electrons’ flow. The positive values of ΔN for both inhibitors indicate that the flow of electrons takes place probably from the inhibitors towards the metal. Also, it is generally assumed that if ΔN<3.6 the inhibition efficiency is found to amplify as the values of ΔN increase [36].According to Lukovits’s study [37], if ΔN < 3.6 then the inhibition efficiency increased with increasing electron-donating ability at the metal surface. The obtained values of ΔN reported in Table 1 are all below 3.6 and PRQ and HCQ have higher values of ΔN than ADQ, CLQ and QNN respectively. This result implies good disposition of PRQ and HCQ molecules to donate their electrons leading to increase their adsorption on the metal surface and to increase their inhibition efficiencies.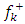 value is maximum and
value is maximum and 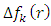 is positive whereas the site for electrophilic attack will be the site where
is positive whereas the site for electrophilic attack will be the site where 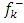 is maximum and
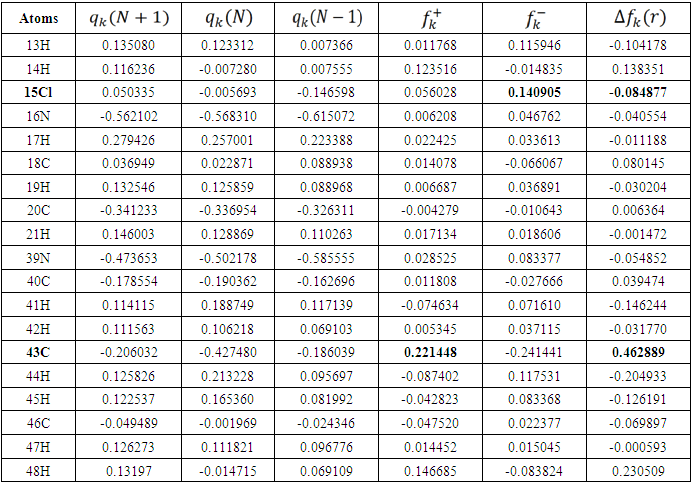
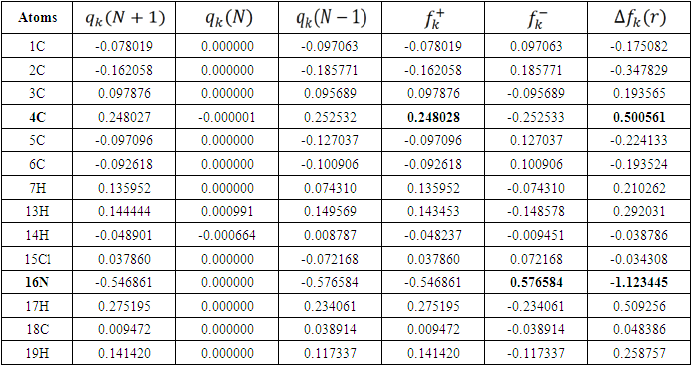
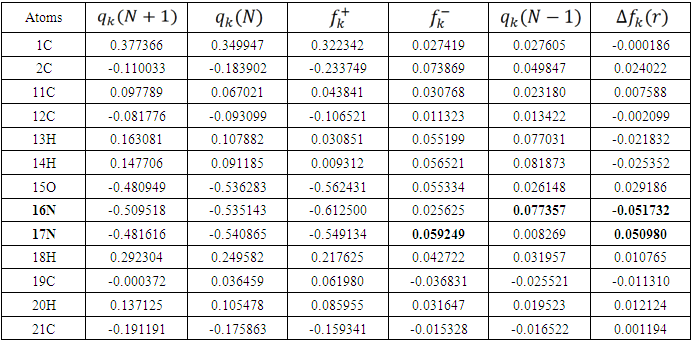
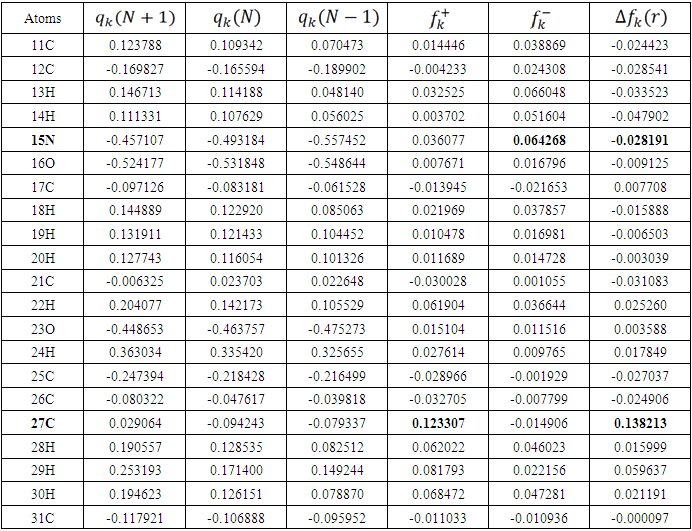
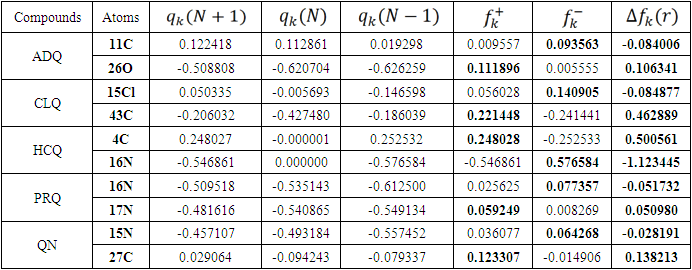
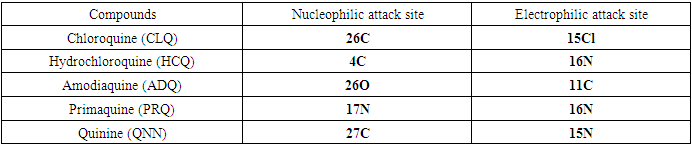
is maximum and 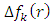 is negative. It has been reported in the literature that the dual descriptor is more accurate local reactivity descriptor than Fukui function [38]. Although, the Fukui function has the capability of revealing nucleophilic and electrophilic regions in a molecule, the dual descriptor is able to unambiguously expose truly nucleophilic and electrophilic regions. Moreover, dual descriptor is less affected by the lack of relaxation terms than the Fukui function [39]. Local parameters calculated through Fukui functions and dual descriptor for different molecules are listed in the tables 1, 2, 3, 4, and 5 below. These sites constitute electronic exchange center where the molecule can lose or gain electrons. From the light of the result given in table 2, 3, 4, 5 and 6, we can summarize the various probable attack sites for each compound studied in Table 7.
is negative. It has been reported in the literature that the dual descriptor is more accurate local reactivity descriptor than Fukui function [38]. Although, the Fukui function has the capability of revealing nucleophilic and electrophilic regions in a molecule, the dual descriptor is able to unambiguously expose truly nucleophilic and electrophilic regions. Moreover, dual descriptor is less affected by the lack of relaxation terms than the Fukui function [39]. Local parameters calculated through Fukui functions and dual descriptor for different molecules are listed in the tables 1, 2, 3, 4, and 5 below. These sites constitute electronic exchange center where the molecule can lose or gain electrons. From the light of the result given in table 2, 3, 4, 5 and 6, we can summarize the various probable attack sites for each compound studied in Table 7. Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML