-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
International Journal of Materials and Chemistry
p-ISSN: 2166-5346 e-ISSN: 2166-5354
2024; 14(3): 49-56
doi:10.5923/j.ijmc.20241403.03
Received: Jun. 27, 2024; Accepted: Jul. 19, 2024; Published: Jul. 20, 2024

Sorption Characteristics of the Adsorbent in the Composition of Bentonite and Opocavid Clays
Nodir Boyjanov1, Kamar Serkayev2, Madina Khamidova2, Shoirakhon Isroilova2
1Urganch State University, Urgench, Uzbekistan
2Tashkent Institute of Chemical-Technology, Tashkent, Uzbekistan
Correspondence to: Nodir Boyjanov, Urganch State University, Urgench, Uzbekistan.
| Email: |  |
Copyright © 2024 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

The physicochemical modification of the surface of natural mineral sorbents is carried out to regulate clays' adsorption and ion exchangeability, and the formation of meso- and microporous structures using specific effective activation methods. The activity of the obtained adsorbents is determined by their ability to absorb gases. However, bleaching of vegetable oils, due to the presence of a complex of large-sized (gossypol-21 Å, phospholipids-30 Å, chlorophyll-15 Å, carotene-170 Å) coloring substances that are not sorbed in meso- and micropores of typical activated clays, proceeds inefficiently. The article proposes a composite composition of inorganic natural minerals of selective action, created taking into account the size of coloring substances of vegetable oils, consisting of bentonite and flake clays, the sorption properties of which differ from traditional adsorbents used in the fat and oil industry.
Keywords: Bentonite, Flake clays, Porosity, Thermal activation, Pore volume, Relative surface
Cite this paper: Nodir Boyjanov, Kamar Serkayev, Madina Khamidova, Shoirakhon Isroilova, Sorption Characteristics of the Adsorbent in the Composition of Bentonite and Opocavid Clays, International Journal of Materials and Chemistry, Vol. 14 No. 3, 2024, pp. 49-56. doi: 10.5923/j.ijmc.20241403.03.
Article Outline
1. Introduction
- The analysis indicated that the structure of clays undergoes significant changes during the production of activated clays. This process relies on the active centers and structural characteristics of the clays, the field of application, and the need to obtain a sorbent with suitable pore size and sorption properties. However, there are differing views on the general scope of clay activation. Consequently, the produced activated clays perform well in bleaching oils of a specific composition but do not achieve the expected results in whitening oils of another composition.The properties of substances to be removed from vegetable oils, the polarity of the ions present, and the size of their particles are of decisive importance. This is because sorption processes, which are based on adsorption through ionic bonds and retention in pores, directly depend on the interaction forces between the adsorbent and adsorbate and the size and charge of the particles to be retained.To address this issue, it is advisable to comprehensively analyze the nature of the coloring substances in vegetable oils and thereby choose the appropriate processing method for the activation of clays. This will help create activated clays with the necessary absorbent properties.
2. Literature Analysis
- The reserves of naturally dispersed minerals are extensive; among them, crystal structures with unique sorption properties are particularly significant. However, their potential is only partially utilized due to the insufficient scientific understanding of the processes required to obtain highly effective sorbents from them, especially bleaching clays, which are effectively used in the oil industry [1].Natural mineral sorbents refer to rocks and minerals with high adsorption or ion exchange properties [2-4]. These include natural zeolites, siliceous minerals (opoka), and clays (such as bentonite clay and palygorskite) [5]. According to the literature, the use of zeolite rocks and siliceous clays as sorbents is less common compared to bentonite, palygorskite clays, and vermiculites.One of the main applications of natural sorbents is in environmental protection and public health. They are used in large water bodies in settlements, drinking water treatment stations, food industry enterprises, and for wastewater treatment. Additionally, these sorbents are employed in oil and gas chemistry, oil processing, and other industries, as well as addressing various agricultural problems [6-9].The sizes of the micropores in natural sorbents are comparable to the sizes of adsorbed molecules, allowing the microporous adsorbent-adsorbate system to be considered single-phase. Adsorption in micropores involves a mechanism of volume filling of pores.The mesopores of sorbents have a developed relative surface, where monomolecular adsorption initially occurs, followed by polymolecular adsorption, with the pores being filled through the mechanism of capillary condensation. The adsorption value of macropores in sorbents is usually ignored because macropores are considered to serve primarily as transport corridors for delivering the adsorbate to the micro- and mesopores of the adsorbent.According to the nature of adsorption and the type of porosity, zeolite, bentonite, and palygorskite clays mainly exhibit ion exchange and, to a lesser extent, molecular adsorption. These clays typically have a micro- and mesoporous structure. In contrast, molecular adsorption is observed in diatomite and siliceous clays, which have meso- and macro-porous structures. Natural sorbents have the ability to absorb molecules of various substances that do not exceed the size of their pores.The geometry and spatial location of the pores serve as the basis for geometric modeling of sorbents. The main models of the geometric structure of pores include globular pores, pores between round discs, pores between polygons, slit-like pores, pores between round rods, and cylindrical capillary pores, which can be open, closed, or serve as barriers and corridors [10]. The group of amorphous sorbents is dominated by siliceous clays with high adsorption properties.Upon heating, dehydrated zeolite and siliceous clays can adsorb molecules of various substances from gas and liquid phases. The primary mechanism in these clays is molecular adsorption, driven by hydroxyl groups on the relative surface and active centers of the siliceous clays [11-16]. Siliceous clays, or opokas, are light, porous rocks composed mainly of small (less than 0.005 mm) particles of silicon dioxide (silica). The amount of active silicon oxide in them ranges from 40% to 80%, with cristobalite and tridymite as the primary forms of active silicon oxide [13].The leading indicators of the quality of opoks are their sorption properties and the percentage of mineral components. Unlike the micropores (active pore diameter 3–6 Å) of bentonites and zeolites, which have a rigid crystal lattice, opoks have meso- and macro-porosities (active pore diameters 20–110 Å) [2].According to their structural characteristics, opal-cristobalite rocks are mixed porous sorbents and are divided into three suitable groups [2,17]. M. G. Ivanov, O. B. Likhareva, A. I. Matern, and H. M. Yaroshevskaya studied the sorption properties of the natural opal-cristobalite opok from the Krasnogvardiya deposit in the Sverdlovsk region. Their research determined that this rock, based on its physical and chemical properties, is a promising sorption material. The authors modified the opok using thermal, chemical, and silicon-organic compounds. By processing the opok with silico-organic modifiers, they were able to create new materials with valuable sorption properties. They conducted a comparative analysis of the physicochemical properties of natural and modified opoks.Their findings indicated that both acidic and alkaline activation led to chemical changes in the mineral compound. Acidic activation increased the relative surface area from 130 m²/g to 138 m²/g due to the leaching of metal cations from the sample. In contrast, alkaline activation decreased the relative surface area from 130 m²/g to 81 m²/g.In a subsequent experiment, heat treatment of the sample at 200°C increased the relative surface area from 130 m²/g (natural opok) to 142 m²/g due to the release of physically bound water. However, increasing the temperature to 400°C and 800°C caused the relative surface area to decrease to 136 m²/g and 114 m²/g, respectively. Following this, formaldehyde sorption was carried out on modified opok, and optimal modification regimes were established. A technological scheme for obtaining sorbent for formaldehyde was proposed [18-19].The authors observed that the activation of opok clays from the Qilachevo and Singeley deposits with acid and alkali led to a decrease in the relative surface area. However, the total volume and diameter of the pores increased. This is explained by the redistribution of pore sizes, with a decrease in micropores and an increase in mesopores [5].A. V. Kondrashova (Saratov mine) conducted differential thermal analysis (DTA) to study the structural changes of dispersed ore (silica) under the influence of temperature. The effect of incineration temperature on the adsorption properties of natural mineral opok was also considered [20-24].N. V. Padalkin and P. N. Ivshinlar studied modified sorbents based on opok for natural and wastewater treatment. They noted that natural mineral-light, finely porous, yet dense siliceous rocks can be used as sorbents after thermal treatment for the purification of natural and waste waters from phosphorus, iron, manganese, aluminum, and other contaminants [25-27].The physicochemical properties, relative surface area, sorption, and other properties of natural aluminosilicates have been studied by scientists in our republic [28]. However, their catalytic properties have yet to be extensively explored.M. Z. Zakirov [29] and V. M. Ligushe studied the catalytic activity of various kaolin and refractory clays (Lyangar, Angren, Sulyukta, Qizil-Kiya) and bentonite clay (Keles, Karmana, Qiziltepa). It was found that bentonite clay deposits, such as those in Kyziltepa (Azkamar) and other locations in Uzbekistan, are of practical importance for the oil industry.In research conducted by O. K. Rahmonov and S. V. Mamadaliyeva, information was provided on the effect of various factors on the sorption properties of local clay minerals, such as Navbahor alkaline earth bentonite, Karmana mine opacified clay, and Tul-Sok palygorskite during mechanical and chemical activation. The results of experiments studying the effect of mechanical activation on the adsorption properties of selected clays were presented. By using the method of mechanical activation, the sorption activity of adsorbents was increased by 1.3 to 1.5 times. The highest degree of paraffin purification (99.3%) was achieved from the composite obtained by mixing activated bentonite and palygorskite clay in a ratio of 1:1, noting that such efficiency was not observed when each adsorbent was used separately.Additionally, information was provided on the occurrence of ultrasonic vibrations, wave propagation, and their use mechanisms in the contact cleaning of paraffins with the composition of adsorbents. This included details on the rational organization of this process and the uniformity and stability of clays used in the composite under the influence of ultrasound [30-32].In Uzbekistan and adjacent areas, there are several deposits of opal and opaline rocks. The Karmana mine, which features opaline clays within siliceous rock developed by M.Z., is located 16 km southwest of the railway station. Layers of clay, ranging from 28 to 46 meters thick, are tentatively identified as sediments forming the northwestern flank of the Zirabulok-Ziaetdin Mountains. Under the microscope, the rock exhibits a uniform structure and texture. At high magnification, one can observe a uniform distribution of carbonate clay (calcium-montmorillonite), an admixture of opal and amorphous silicon oxide, and very little quartz throughout the rock [33]. Additionally, E.G. Libenzon (1968) noted the presence of zeolite in the Karmana opaque clays [33].Another significant deposit, the Ziaetdin-Zirabulak mine, is located southwest of the Ziaetdin-Zirabulak-Tashkent railway, approximately 20 km away. The raw material layer averages 8 to 15 meters in thickness and shares analogous macroscopic and microscopic characteristics with the Karmana deposit [33].Siliceous rocks in Uzbekistan can be classified into two types:1) Opoka deposits lacking carbonate and clay mineral mixtures (such as Mullali, Darbaza, and Keles mines);2) Mixtures of opaque clays containing 10 to 35% carbonate (such as the Karmana, Zirabulak-Ziaetdin, and Beshbulok deposits).The second type, opaque clays, have received less attention despite their potential as clays with high hydraulic and adsorption activity. Research indicates they should be classified as such based on their mineralogical composition, which includes amorphous silica (5-10%), cristobalite (opal), montmorillonite, dolomite, calcite, and zeolite mixtures. The chemical composition of natural opaque clays also includes these oxides, differing in quantity (see Table 1), influencing the size and volume of their pores during activation.
|
3. Research Methods
- Based on previous research results, the composite preparation involves a three-step process. Initially, natural calcium bentonite from the Navbahor mine was activated using an acidic method under laboratory conditions. In prior experiments, hydrochloric acid (HCl) was found less effective, resulting in adsorbents with passive properties and residual chlorine ions. Therefore, a 15% concentrated sulfuric acid solution was chosen, achieving optimal results. The clay-to-acidic solution ratio (hydromodule) was set at 1:2.5, with acid consumption ranging from 25% to 50% [35].In the second stage, Karmana natural opaque clay was crushed in an aluminum mortar. The portion passing through a 1 mm sieve was thermally treated at 200°C for 2 hours in a Nevo-QTZ 110/7 model muffle furnace to enhance its adsorption capacity. Thermally activated clay samples were further crushed until 90% passed through a 56 μm sieve and stored hermetically.For the third stage, 10%, 20%, and 30% of the sample prepared through thermal activation of natural opaque clay was added to the acid-activated bentonite clay samples. Subsequently, physicochemical analyses were conducted on the finished adsorbent samples.To compare sorption characteristics, Super-FF brand clay from Pakistan, used in oil enterprises, served as a control sample. The B.E.T. (Brunauer-Emmet-Teller) method was employed using Micromeritics Gemini VII Surface Area and Porosity Analyzer and Quantachrome® ASiQwin™ Automated Gas Sorption Data Acquisition and Reduction, Quantachrome Instruments version 5.21 equipment to determine the relative surface area and pore size of the samples. This method, which calculates adsorption isotherms based on the adsorbent's adsorption level at constant temperature (r/r0) under varying pressure, remains the most widely used today. It considers the cross-sectional surface of the monolayer and the adsorbed gas molecule to compute the relative surface area. The analysis included assessing pore volume and size, crucial for characterizing the adsorption properties of bleaching clays.The determination principle involved measuring the volume of adsorbate gas absorbed during cooling (adsorption) and heating (desorption) phases using a steady flow of helium-nitrogen with specified composition through the adsorber containing the sample.
4. Results and Discussion
- Based on the findings from the aforementioned studies, which included the analysis of vegetable oil composition and physico-chemical properties of bentonite from the Navbahor mine in comparison with other activated clays, it was concluded that existing perspectives on clay activation need revision [35].The analyses revealed that while numerous studies have achieved specific outcomes, the structural transformations during the production of activated clays for vegetable oil refining are broad. Depending on the active centers and structural composition of clays, the application area necessitates tailored processing to obtain sorbents with requisite pore sizes and adsorption properties. Views on the general applicability of clay activation methods thus need reconsideration. Consequently, activated clays perform effectively in bleaching oils of specific compositions but may not yield anticipated results in others.To address these issues, comprehensive analysis of the nature of coloring substances in vegetable oils is recommended, enabling selection of appropriate clay activation methods to tailor activated clay structures with optimal adsorptive properties. The characteristics of substances to be removed from vegetable oils, including polarity of ions and particle size, are critical. Sorption processes involving adsorption via ionic bonds and retention in pores directly hinge on interaction forces between adsorbent and adsorbate, as well as particle size and charge.Our observations indicate that bentonite clays excel in absorbing free fatty acids from vegetable oils, whereas opaque clays effectively absorb metal and soap residues. Acid or alkali activation enhances sorption properties across all bentonite clays, whereas using opaque clays solely through thermal activation holds significant ecological and economic importance.Harmful additives in vegetable oils, such as gossypol (21 Å), phospholipids (30 Å), chlorophyll (15 Å), and carotene (170 Å), are substances with high molecular sizes. Current research on opaque clay indicates pore sizes greater than 20 Å, suitable for removing substances smaller than 20 Å like chlorophyll, while microporous bentonite clay is effective for secondary products formed during processing.Literature analysis suggests that despite the excellent sorption properties and porous structure of opaque clays, their individual use as sorbents may yield different results due to the complex composition of vegetable oils. To address this, a selectively acting composite is proposed based on acid-activated bentonite clay and opaque clay. This composite takes into account the polarity and particle size of ions present in the oils. Each clay component in the composite exhibits selective absorption properties: bentonite clays absorb small-molecule pigments and fatty acids, while opaque clays target large-molecule substances like gossypol, metal ions, and soap residues.In this study, effective methods involving physical and chemical modification of natural mineral sorbents are employed to regulate their adsorption and ion exchange capacities. Taking these factors into consideration, we proposed a composition comprising inorganic natural minerals, specifically bentonite and opaque clays. This composition exhibits selective sorption properties that differ from traditional adsorbents commonly used in the oil industry.Therefore, varying the type of activator enables the creation of a porous structure with a wide range of micro, meso, and macropores, facilitating selective sorption of molecules of varying sizes. This approach allows for predicting the adsorption properties of substances with diverse molecular sizes.Literature analysis indicates that acid and alkaline activation of opaque clays did not yield anticipated improvements in their absorption properties. Based on this, thermal treatment alone was found to be sufficient. Experimental studies identified optimal temperatures for the process, as illustrated in Figure 1.
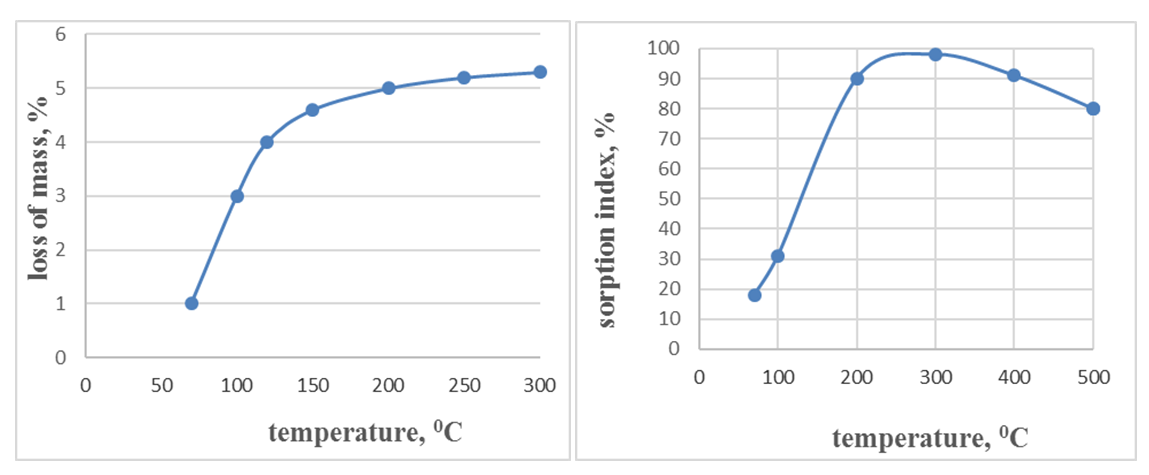 | Figure 1. Illustrates the effect of temperature during heat treatment on mass loss (a) and sorption parameters (b) of opaque clay |
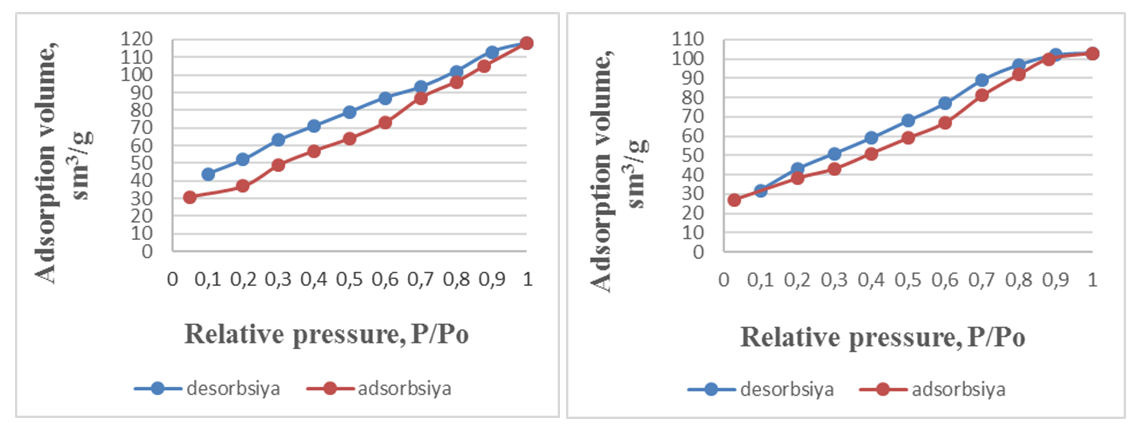 | Figure 2. Illustrates the adsorption-desorption isotherms of thermally activated natural opaque clay (a) and composite clays derived from bentonite and opaque clays (b) in a nitrogen environment |
|
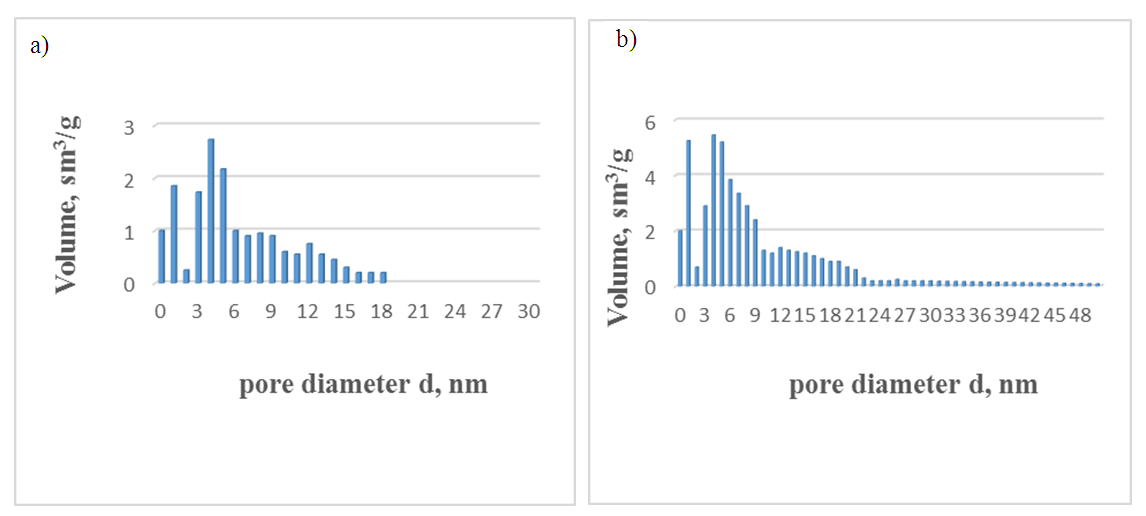 | Figure 3. The size distribution of pores in natural (a) and acid-activated (b) bentonite clays |
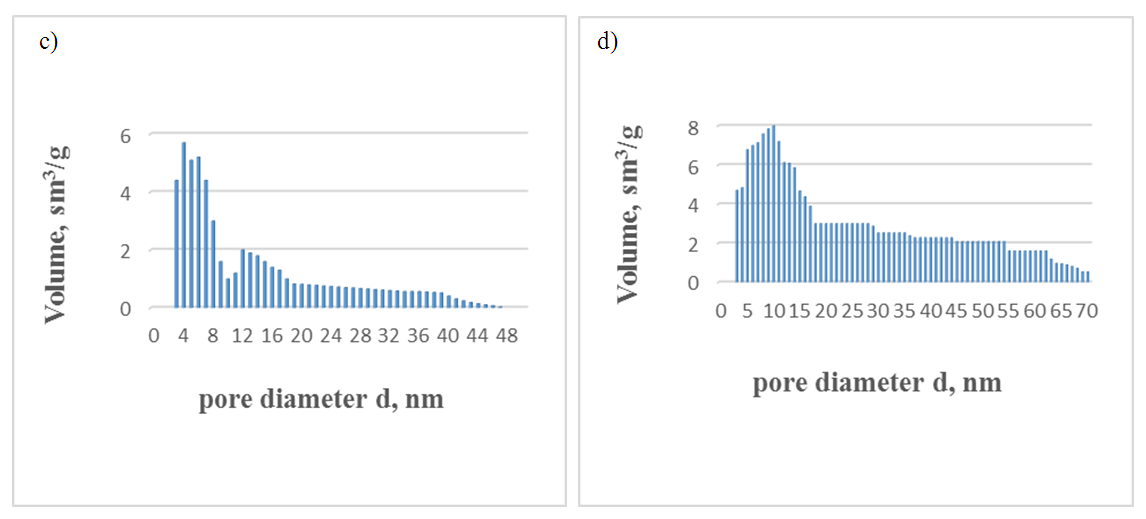 | Figure 4. Control (c) Super F.F. and thermally activated opaque clay (d) pore size distribution |
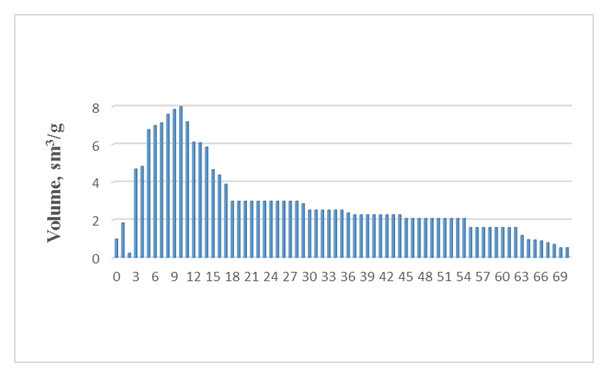 | Figure 5. Size distribution of pores in composite clay, pore diameter d, nm |
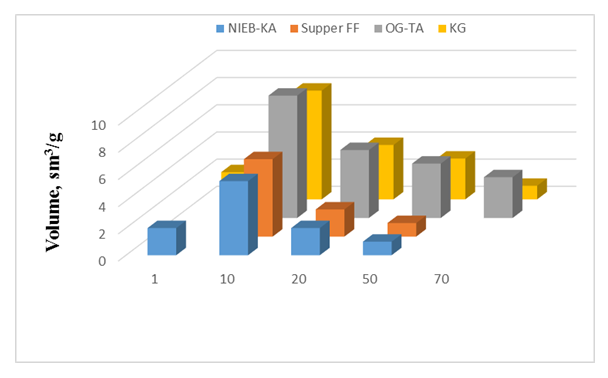 | Figure 6. Comparison of available porosity in NIEB-KA, Super FF, OG-TA, KG |
5. Conclusions
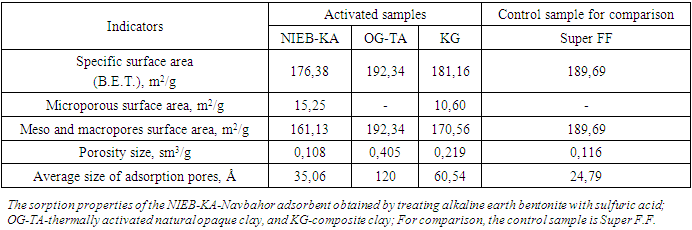
- Acid-activated bentonite clay features micropores up to 2 nm and a small number of mesopores ranging from 2 nm to 50 nm, similar to the imported Super F.F. brand Pakistani clay. In contrast, the active pores in the opaque clay included in the composite range from 3 nm to 70 nm (30-700 Å), with an average size of 12 nm (120 Å), and lack micropores smaller than 3 nm. Experimental evidence indicates that increasing mesopores in acid-activated clay enhances its effectiveness.The recommended composite adsorbent for pigment removal from vegetable oils in the oil industry boasts a relative surface area similar to that of the control sample, but offers higher pore volume and larger average pore size, resulting in reduced pigment content in oils. The presence of microporous bentonite clay in the composition is crucial due to its size and ionic bonding properties. Therefore, this composite adsorbent holds a significant advantage over traditionally used clays for vegetable oils with relatively high levels of pigmentation.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML