-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
International Journal of Materials and Chemistry
p-ISSN: 2166-5346 e-ISSN: 2166-5354
2024; 14(3): 37-48
doi:10.5923/j.ijmc.20241403.02
Received: May 17, 2024; Accepted: Jun. 8, 2024; Published: Jun. 20, 2024

Isotherms, Kinetics and Thermodynamic Investigation of the Adsorptive Capacity of Chrysophyllum Albidum Seed Shell an Agro-Waste for the Removal of Heavy Metals in Synthetic Wastewater
Osundiya Medinat Olubunmi1, Olowu Rasaq Adewale1, Onifade Olayinka Omoniyi2, Elesho Adeseye Omololu1, Osifeko Olawale Lawrence1, Oyewole Toyib Seun1, Tovide Oluwakemi Omotunde1, Balogun Sunmisola Rukayat1, Adejare Adeniyi Ayemu1, Anko Senami Oluwafunmilola1, Shotonwa Ibukun Oluwaseun1, Onwordi Chionyedua Theresa1
1Department of Chemistry, Lagos State University, Ojo, Lagos State
2Department of Biochemistry, College of Medicine University of Lagos, CMUL Idi-araba, Lagos State
Correspondence to: Olowu Rasaq Adewale, Department of Chemistry, Lagos State University, Ojo, Lagos State.
| Email: |  |
Copyright © 2024 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

The discharge of heavy metals into the environment from industrial wastes is a major problem that needs immediate attention. This study investigated the potential of Chrysophyllum albidum (African star apple) seed shells as an adsorbent for the removal of Mn2+, Co2+ and Cr6+ ion from synthetic wastewater. The seed shell was washed, dried, crushed and sieved, and this was subsequently used for possible adsorption process. Batch adsorption technique was adopted to examine the effects of temperature, pH, contact time, adsorbent dosage and initial metal concentration on the adsorption of Mn2+, Co2+ and Cr6+ ion from synthetic wastewater at 25°C and optimum pH of 6.68 with equilibrium contact time of 60 minutes. Investigation of the surface morphology of the adsorbent before and after adsorption using scanning electron micrograph (SEM) showed the particulate arrangement, pore spaces indicating that metals were adsorbed on the adsorbent. The adsorbent was probed with Fourier Transformed Infrared Spectrometer (FTIR) which revealed the presence of alcohol (O-H), amide (NH2) alkanes (-CH2- and -CH3) and carbonyl (C=O) groups at a frequency band of 3645.78 cm-1, 3458.63 cm-1 1391 and 1100 respectively confirming the potential mechanism of adsorption across different functional groups. The adsorption kinetics uptake for Mn2+, Co2+, and Cr6+ at various initial metal ions concentrations was analysed using pseudo-first-order and pseudo-second-order. The adsorption process fitted with the pseudo-second-order which showed that the metals were chemisorbed on the biosorbent surface. The percentage adsorption of Mn2+, Co2+ and Cr6+ ions ranges from 76.90 - 84.81, 55.99 - 64.50 and 49.88 - 54.81 respectively. The adsorption data were fitted to the Langmuir adsorption isotherm better than Freundlich models. Solution pH and temperature significantly influenced the adsorption process and a negative value of ∆G° was obtained which suggest that the sorption process was spontaneous and feasible. This showed that the biomass of Chrysophyllum albidum seed shell (CASS) is a promising cost-effective biosorbent substitute in the remediation of heavy metals contamination in wastewater.
Keywords: Chrysophyllum albidum seed shell, Thermodynamic, Kinetics, Adsorption isotherms, Heavy metals
Cite this paper: Osundiya Medinat Olubunmi, Olowu Rasaq Adewale, Onifade Olayinka Omoniyi, Elesho Adeseye Omololu, Osifeko Olawale Lawrence, Oyewole Toyib Seun, Tovide Oluwakemi Omotunde, Balogun Sunmisola Rukayat, Adejare Adeniyi Ayemu, Anko Senami Oluwafunmilola, Shotonwa Ibukun Oluwaseun, Onwordi Chionyedua Theresa, Isotherms, Kinetics and Thermodynamic Investigation of the Adsorptive Capacity of Chrysophyllum Albidum Seed Shell an Agro-Waste for the Removal of Heavy Metals in Synthetic Wastewater, International Journal of Materials and Chemistry, Vol. 14 No. 3, 2024, pp. 37-48. doi: 10.5923/j.ijmc.20241403.02.
Article Outline
1. Introduction
- Pollutants have become a severe issue to be dealt with in all developing countries, and Nigeria is not an exception, especially due to the consistent contamination of the environment since independence till date, leading to adverse threats on plant, animal, and human life [1]. Cadmium, manganese, cobalt, chromium, lead, nickel, mercury, arsenic and many more have been established as heavy metals as they have relatively high densities, atomic weight and atomic numbers, and are ubiquitous in the environment [1-3]. Most of the heavy metals are harmful to life due to their toxicity and bioaccumulation even at low concentrations [1,4]. Heavy metals get into the environment via various ways which are, the anthropogenic and non-anthropogenic sources [5-6]. The anthropogenic sources include tanning, laundry, dying, metal cleaning, mining, cement manufacturing, pulp and paper and chemical manufacturing processes, while non-anthropogenic sources include volcanic eruption and weathering of metal-containing rock. The different types of contamination and pollution of water sources with toxic heavy metals are of major concern. Heavy metals are resistant against micro-organisms which are known to decompose some organic pollutants, therefore, such pollutants cannot be removed naturally from the environment [5-6]. Among all forms of pollution, heavy metals have been identified as one of the main threats endangering human and wildlife causing brain damages, disrupting the circulatory system, as well as disrupting the endocrine system. Hence, these are called endocrine disrupting chemicals [5-7].Adsorption techniques are effective and efficient for removing pollutants such as heavy metals, organic pollutants and dyes from wastewater [1,3-5]. Activated carbon is highly competent and valuable, but its high cost and difficult regeneration limits its use. Therefore, low cost, renewable, and easily available materials as adsorbents are needed, and attention has been focused on developing them, which includes agricultural and forestry by-products such as walnut shell, egg shell, wheat straw, rice and peanut husks, sawdust, etc. [5-9]. The processes of heavy metals removal from aqueous solutions are physical, chemical and biological processes and some of these involve precipitation, electrolysis, ion exchange, coagulation, foam flotation, filtration, sedimentation, solvent extraction, adsorption, chemical oxidation, disinfection and chemical precipitation methods [10-11]. However, adsorption is considered superior compared to some of the other methods of heavy metal removal because of its convenience, easy operation and simplicity of design [12]. This process creates a film of the adsorbate on the surface of the adsorbent and it differs from absorption, in which a fluid is dissolved by a liquid or solid respectively. The characteristics of adsorption that effects the removal of contamination are adsorption capacity, specific surface area, pore volume, grain size and pore size distribution [13-14]. A variety of adsorbents are used and studied in the field of pollutant removal from water resources like activated carbon, chelating materials, zeolites, bio-adsorbents and etc. Nonetheless, polymer based adsorbents are used widely in this respect due to their low cost, stability, high durability and high efficiency [15-18]. Agricultural wastes are now considered an alternative and important precursors for the production of adsorbent because of their low cost and they are mainly composed of highly fibrous ligno-cellulosic materials with relatively large surface areas that can provide intrinsic adsorptive sites to many substrates [1,19-28]. They are renewable and abundantly available at little or no cost for adsorption of both inorganic and organic pollutants thereby providing way of recycling agricultural wastes which could constitute environmental nuisance [1,5,28-39]. The study is aimed at the use of Chrysophyllum albidum (African star apple) seed shells as an adsorbent for the removal of Mn2+, Co2+ and Cr6+ ion from synthetic wastewater.
2. Material and Methods
2.1. Materials
- The chosen precursor material, Africa star apple seed shell, an agro-waste, was collected at a local market in the arena area of Oshodi, Lagos State, Nigeria. The seeds were washed and rinsed with deionized water to remove the dirt and other extraneous materials. The washed seeds were dried in the laboratory, and then broken to remove the cotyledon. The seed shells were grinded using a mortar and pestle, and sieved with laboratory test sieve of aperture 250 MIC. It was then oven dried at 105°C for 5 hours, cooled in a desiccator and stored in an air tight container. The stock solution of manganese (II), cobalt (II) and chromium (VI) ion (1000 mg/L) was obtained by dissolving their respective water-soluble salts in deionised water, and the working solution was prepared from the stock.
2.2. Characterization
- The physiochemical properties of Chrysophyllum albidum seed shell powder was characterized using Fourier Transform Infrared (FTIR) and Scanning Electron Microscopy (SEM) (a Tecnai G2F2OX-TTwinMAT model). SEM was used for morphology investigation of the adsorbents before and after adsorption while a NicoletTM iS50 FTIR was used to identify the presence of functional groups on the adsorbent surface which can readily bind with Mn2+, Co2+ and Cr6+ ions to enhance their ease of removal.
2.3. Batch Adsorption Experiments
- The adsorption study was conducted by batch experiment under different conditions for a period of time in a test tube. The adsorption study was conducted at room temperature (25°C). The equilibration time for the adsorption of manganese, cobalt and chromium on Africa star apple seed shells was carried out by introducing 1.0 g of pulverized sample into the solution containing the three adsorbate of interest of 20 mg/L in conical flasks at room temperature under optimum pH each to determine the contact time required for the metal ions to reach adsorption equilibrium. The shell suspensions were agitated using mechanical orbital shaker (Model SSMI, SSL1-CCL) at a speed of 150rpm for 30, 60, 90, 120, and 150mins. After adsorption was completed, the solution was then filtered using Whatman filter paper and the filtrate were taken for Atomic Absorption Spectroscopy (AAS) analysis to determine the metal ions left in the solution. The time for metal ion adsorption to reach equilibrium was determined graphically from the plot of adsorption capacity against time of equilibrium. The effects of pH, temperature, adsorbent dose and metal ion concentration were also evaluated using the same procedure described above but, in each case, the pH (2.8, 4.8, 6.8, 8.8, and 10.8), temperature (25, 30, 35. 40 and 45°C), adsorbent dose (0.2, 0.4, 0.6, 0.8 and 1.0 g) and metal ion concentration (5, 10, 15, 20 and 25) mg/L respectively while keeping the other parameters constant so as to obtain the optimum pH for the experiment. The filtrate from each of the above procedures were analysed using atomic absorption spectrophotomer ((Buck scientific model 210) to determine the amount of metal ions left in the solution after adsorption. The percentage removal of metal ion and the amount of ions adsorbed per unit weight of adsorbent was calculated using the formula below
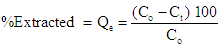 | (1) |
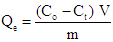 | (2) |
3. Results and Discussion
3.1. Scanning Electron Microscope (SEM)
- The low and higher magnification micrographs images (Figure 1) revealed that the prepared Chrysophyllum albidum seed shell adsorbents have firm, irregular shape and cavities because they exhibits many large pores and rough surface. The cavities found in the micrograph indicate that it consists of tubular vitreous network and wide varieties of pores, and more evidence of large pores on the surface of the prepared adsorbent was observed. The implication of this was that the prepared adsorbent from Chryphyllum albidum seed shell has enough rough and pore spaces for adsorption activities to take place. Therefore, there is a high tendency for the heavy metals adsorption since surface plays a prominent role in the adsorption process [36-37].
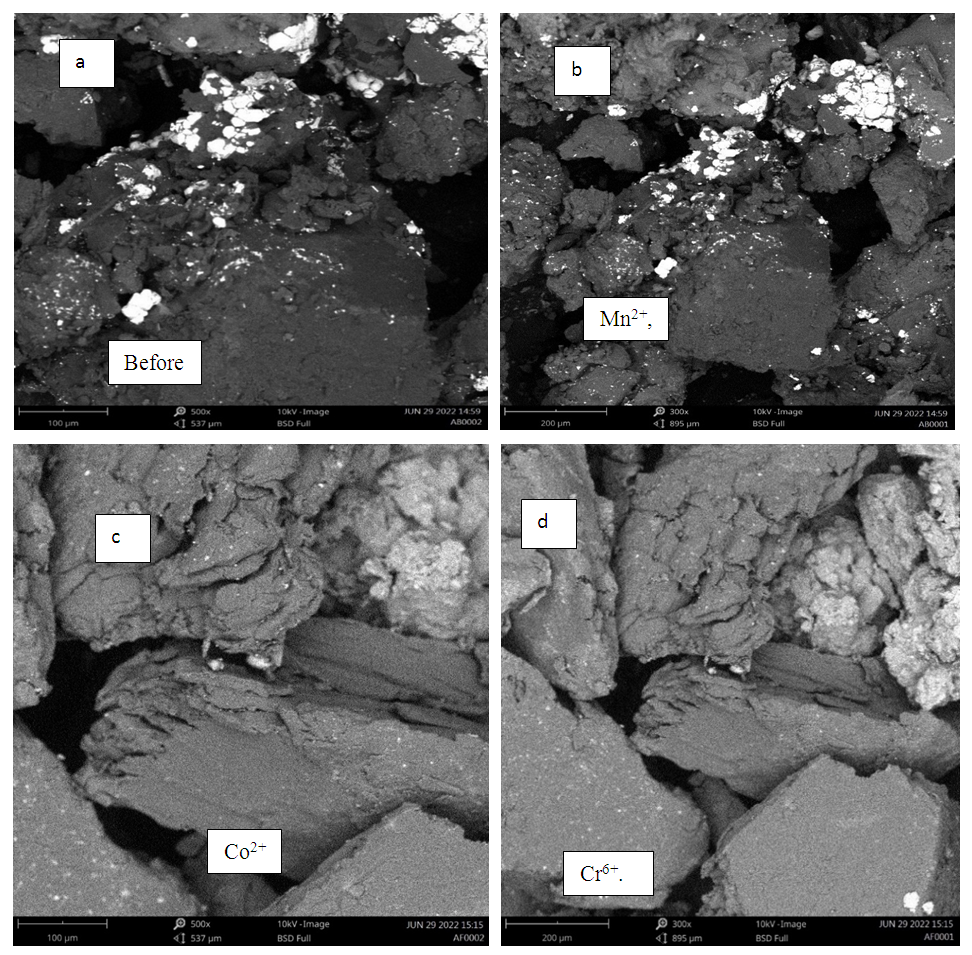 | Figure 1. SEM Images of Chrysophyllum albidum seed shell powdered (a) before adsorption and (b), (c) and (d) after adsorption of Mn2+, Co2+ and Cr6+ respectively |
3.2. Fourier Transformed Infrared (FTIR)
- The FTIR spectrum gives information about the characteristic functional groups on the surface of the sample before and after the adsorption of Mn2+, Co2+ and Cr6+ to ascertain the possible involvement of the functional groups on the surface of adsorbent in the adsorption of metal ions of interest is presented in Figure 2. The FTIR spectra in Figure 2 illustrates the various peaks and identified functional groups for the Chrysophyllum albidum seed shell (CASS) adsorbent; within the specified wavelength range of 4000 cm-1, – 1500 cm-1, the broad peak at 3645.78 cm-1 can be attributed to the presence of vibrational stretching of the bonded –OH group of alcohol or carboxylic group, 3458.63 cm-1 can be assigned to N-H stretching of the amide and that at 3097.2 cm-1 can be assigned to =C-H aromatic. A sharp absorption band at 1698.6 cm-1 can be attributed to –C=C- stretching group usually cyclic alkenes since the region from 2000 to 1500 cm –1 is where double bonds (-C═C, -C═O and -C═N,) absorb, bands at 1391.45 cm-1 can be assigned to the C-H bend of aromatic or cyclic compounds, while the signal at 1100.02 cm-1 indicated the presence of C-O stretching vibration of an alcohol. There are clues that the absorption bands identified in the spectra and corresponding functional groups in the adsorbent could enhance the surfaces on which adsorption would take place.
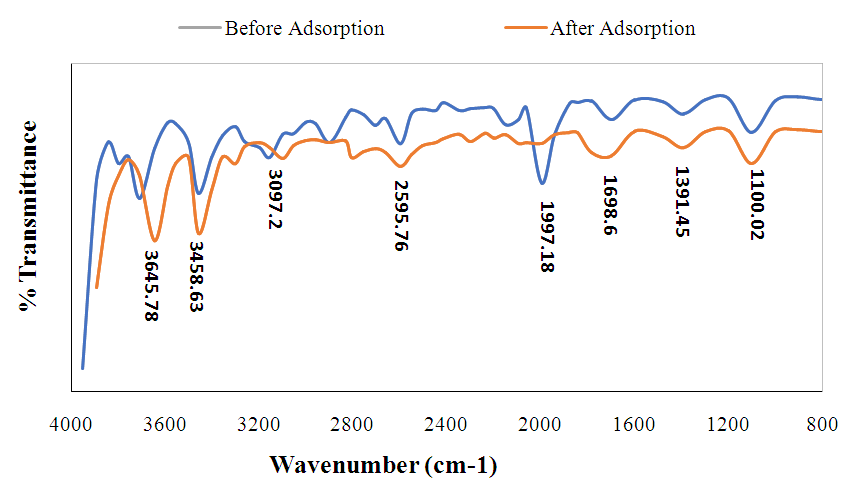 | Figure 2. FTIR spectrum of Chrysophyllum albidum seed shell before and after adsorption |
3.3. Effect of Parameters on Adsorption Studies
3.3.1. Effect of Adsorbent Dosage on Adsorption of Mn2+, Co2+ and Cr6+ onto CASS
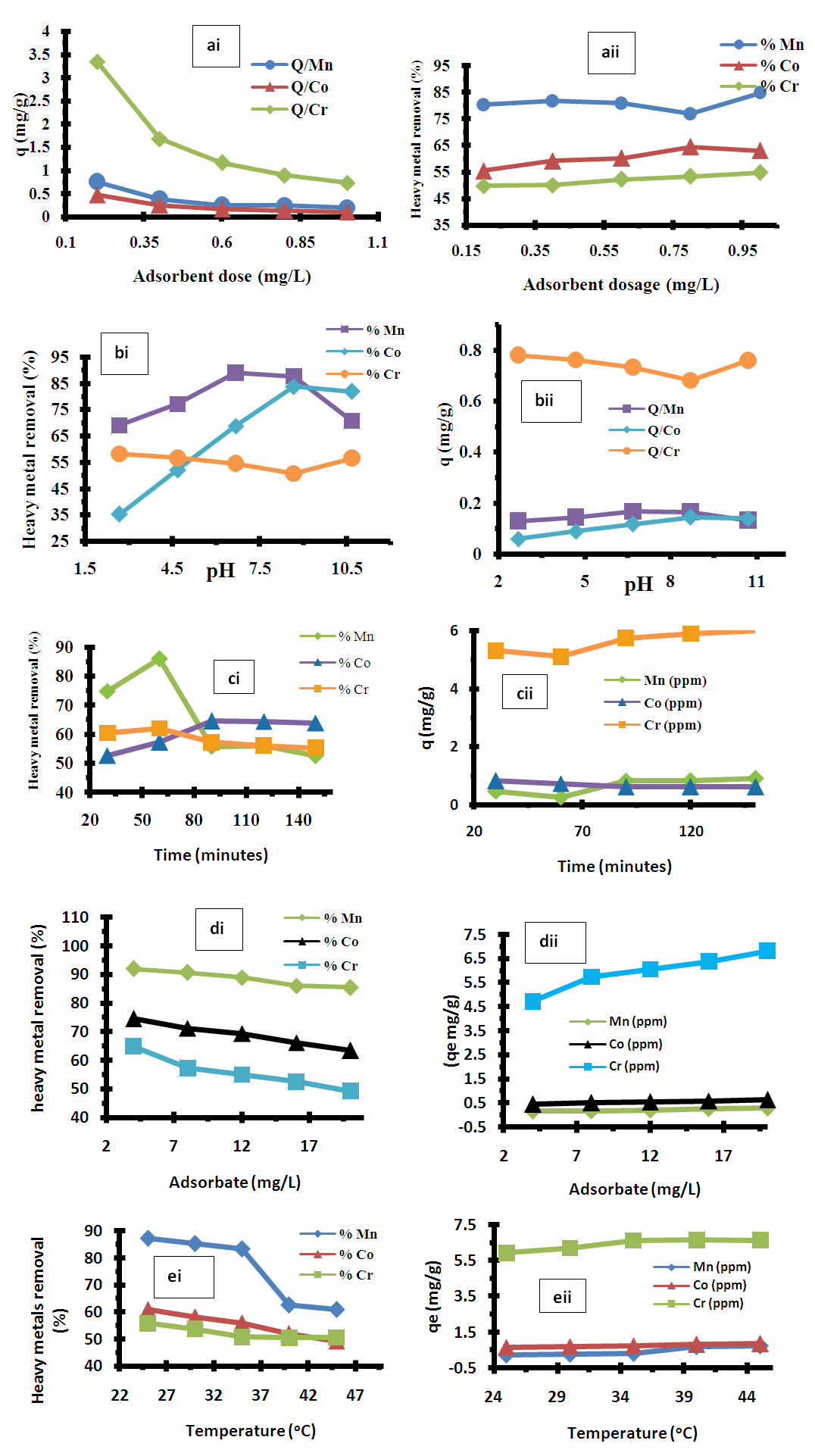
- Figure 3a shows that the limited number of the adsorbing species is present for a relatively larger number of available surface sites on the adsorbent at higher dosages. This supports the facts that at higher dosages of the adsorbent, there would be higher availability of exchangeable sites from metals ions uptake [14]. Adsorption capacity of the prepared adsorbents is high at lower concentrations, as can be interpreted; the sample CASS exhibited a higher adsorption capacity for all metals in the synthetic or polluted wastewater considered. On the other hand, removal efficiency of the metal ions showed narrow variation with the increase of metal concentration and increase of adsorbent dosage. Higher dosage of adsorbent increased the adsorption due to more surfaces sites and functional groups with higher availability of exchangeable sites for metals ions uptake on the adsorbent [15].
3.3.2. Effect of pH on Adsorption Studies
- The pH of the aqueous solution is an important controlling parameter in the adsorption process. It was observed that the pH was affected by the amount of adsorbate as shown in Figure.3b. The percentage removal for the adsorption of manganese (II) ion and cobalt (II) ion increased with increase in pH from 2.68 to 6.68, with percentage removal value of 85% Mn and 80% Co. However, the uptake of these ions decreased slightly from pH of 8.68 to 10.68 indicating that the percentage efficiency was better in the acidic pH ranges. The minimum percentage removal observed at low pH may be attributed to large amount of H3O+ which competed with the positively charged metal ions for the adsorbent surface sites, therefore, the available surface area and subsequent adsorption of the metal ion was reduced. The increase in metal ion removal as pH increases could be explained on the basis of a decrease in competition between hydroxonium ions and metal species for the surface sites and also by the decrease in positive surface charge on the adsorbents, which resulted in a lower electrostatic repulsion between the surface and the metal ions, and hence uptake of metal ions increased. A similar report was proposed by several earlier works for metal adsorption on different adsorbents [15-16].
3.3.3. Effect of Contact Time on Adsorption Studies
- The contact time is one of the most effective factors in batch adsorption processes and it is necessary to determine the contact time to reach equilibrium. The adsorbate adsorption uptake increased as contact time increased from 30-150 minutes, and reached equilibrium at 60 minutes (Figure 3c). The result suggests that, adsorption takes place rapidly at the initial stage on the external surface of the adsorbent followed by a slower internal diffusion process, which may be the rate determining step [12,17]. In addition, the fast adsorption at the initial stage may be due to the fact that a large number of surface sites are available for adsorption but with time, the remaining surface sites are difficult to be occupied due to the repulsion between the solute molecules of the solid and bulk phases, thus, taking longer time to reach equilibrium [17].
3.3.4. Effect of Initial Concentrations on Adsorption Studies
- The effect of initial concentration of the metal ions on the adsorption rate of adsorbate from the synthetic wastewater was studied for the metal ion concentrations ranging from 4 - 20 mg/L. Figure 3d shows the effect of initial concentrations of the adsorbate on percentage removal by adsorbent. The percentage removal decreased with increasing initial metal ion concentrations. The lower uptake at higher concentration resulted from an increased ratio of initial adsorption number of moles of the metal ions to the available surface area; hence fractionally becomes dependent on initial concentration. The initial metal ion concentration provides an important motivating force to overcome the mass transfer resistance of the metal ions between the aqueous and solid phases. Therefore, at higher initial metal ions concentration, the number of ions competing for the available sites on the surface of adsorbent was high, resulting in higher adsorbate adsorption capacity [17-18].
3.3.5. Effect of Temperature on Adsorption Studies
- Temperature is an important parameter that influence metal ions adsorption. The various adsorption tests were carried out at different temperatures ranging from 25°C to 45°C, using 20 mg/L initial adsorbate concentrations at pH 6.68 and with a dose of 1.0 g of adsorbent. As shown in Figure 3e, it was observed that the adsorption capacity decreases more as temperature of the reaction medium increases from 25°C to 45°C, which indicates the exothermic adsorption process of adsorbate onto adsorbent. According to Amuda et al [21], this phenomenon could be explained by the adsorption of adsorbate caused by an increase in the thermal energy available. Increasing the temperature would induce greater mobility of the adsorbate, leading to the adsorption of metal ions [17-18].
3.4. Adsorption Isotherms Studies
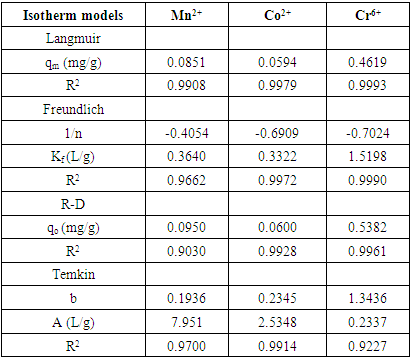
- As presented in Table 1, the adsorption capacity was evaluated as 147.65 mg/g at 35°C, the high R2 values were obtained from all isotherms, whereas the highest value belongs to the Langmuir isotherm with 0.9908, 0.9979 and 0.9993 respectively. The data obtained showed that the better fits were obtained for the Langmuir isotherm model as shown in figure 4. This suggests that the adsorption of Mn2+, Co2+ and Cr6+ occurs in localized sites indicating the occurrence of homogeneous and monolayer adsorption coverage on the used adsorbent. 1/𝑛 value, calculated in accordance with Freundlich equation, indicates whether the adsorption process is spontaneous. Spontaneous adsorption conditions indicate where n values are greater than 1 [27]. In the present study n value was found to be lesser than 1 which is an indication of high adsorption by the adsorbent confirming that chrysophyllum albidum seed shell (CASS) was good at adsorbing the heavy metal. In addition the low value of n also indicates the existence of a high proportion of active site [40].
|
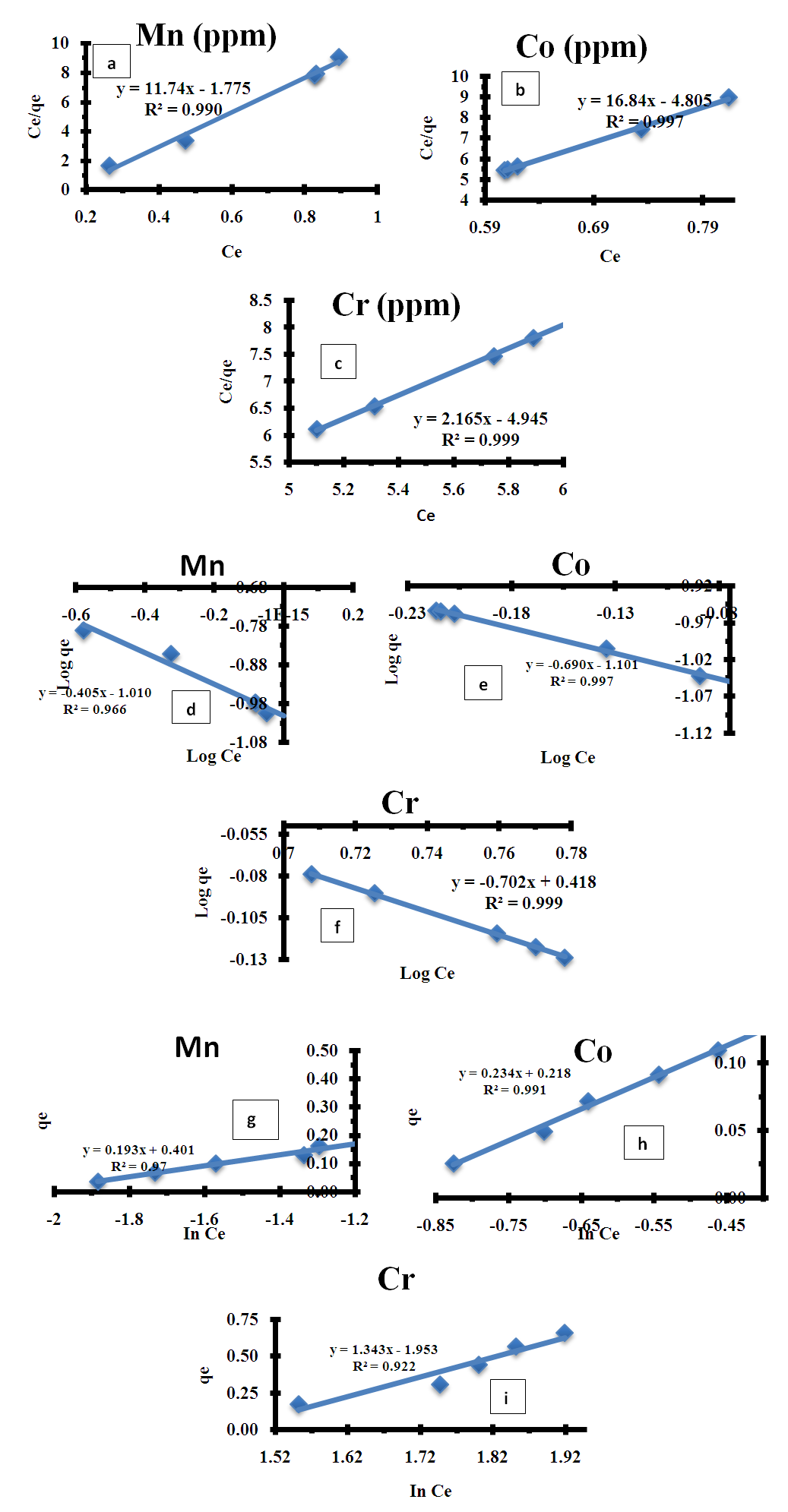 | Figure 4. Adsorption isotherm plot (a,b,c) Langmuir for Mn2+, (b) Co2+, and Cr6+ (d,e,f) Freundlich for Mn2+, Co2+, and Cr6+) and (g,h,i) Temkin Mn2+, (b) Co2+, and Cr6+ |
3.5. Adsorption Kinetics Model
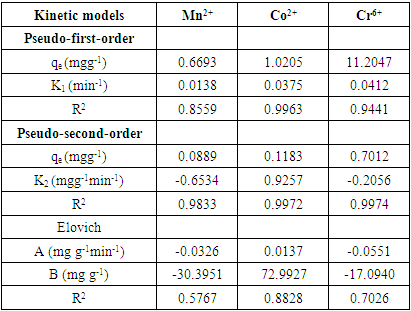
- Pseudo First Order and Pseudo Second KineticsThe study of the kinetics of adsorption is desirable as it provides information about the mechanism and characteristics of adsorption, which is important for efficiency of the process [36-38]. Adsorption kinetic modeling is very useful for better understanding of Mn2+, Co2+ and Cr6+ adsorption mechanisms onto Chrysophyllum abidum. Adsorption kinetics assist in investigating the potential rate controlling mechanism and helps in selecting optimum operating conditions in designing and optimizing full scale applications [36]. In this work, the mechanism of adsorption was investigated by using a variety of kinetic models based on aqueous phase concentrations of Mn2+, Co2+ and Cr6+. Pseudo first order, pseudo second order and Elovich kinetic model were used in the investigation (fig 5). Equation kinetic models were tested at different concentrations in this study to determine which model is in good agreement with experimental 𝑞𝑒 (adsorption capacity) value, thus suggesting the adsorption system model.Pseudo- first- order modelPseudo-first order has the linear form;
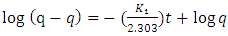 | (3) |
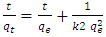 | (4) |
|
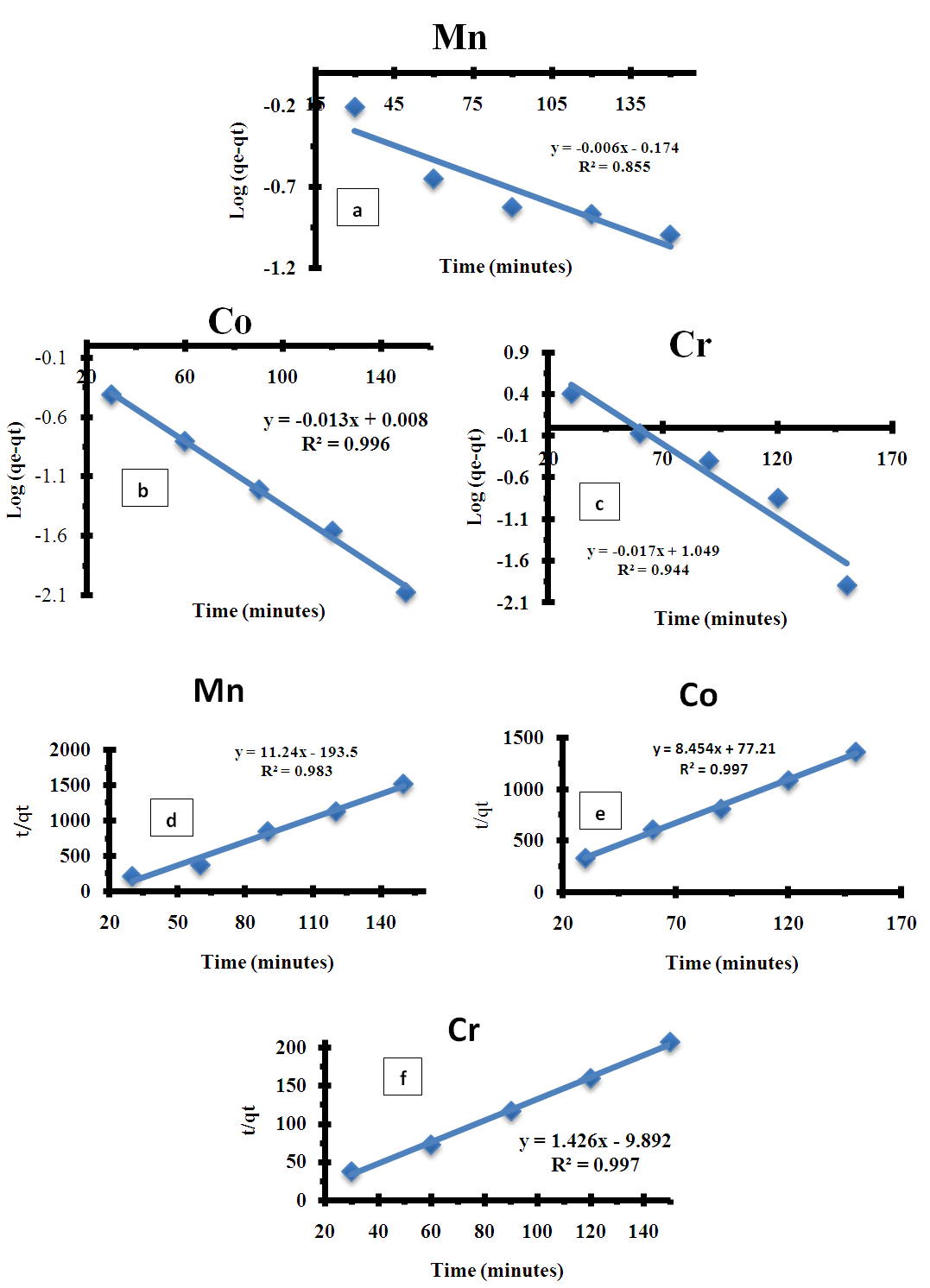 | Figure 5. Pseudo first order plot (a,b,c) for Mn2+, Co2+, and Cr6+ and Pseudo second order plot (d,e,f) for Mn2+, Co2+, and Cr6+ |
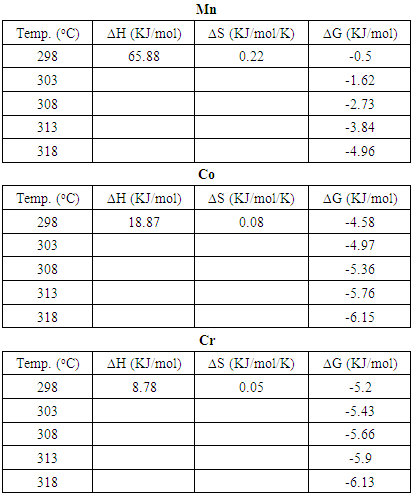
3.6. Thermodynamics Studies
- The thermodynamic data reflect the feasibility and favorability of the adsorption. The parameters such as free energy change (ΔG°), enthalpy change (ΔH°) and entropy change (ΔS°) can be estimated by the change of equilibrium constants with temperature. The values of ΔH° and ΔS° were calculated using the following equation of Van’t Hoff [1,35-38]:
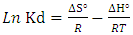 | (5) |
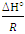 and an intercept of
and an intercept of 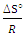 . Then ΔG° is obtained at any temperature from the following equation;
. Then ΔG° is obtained at any temperature from the following equation;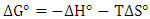 | (6) |
|
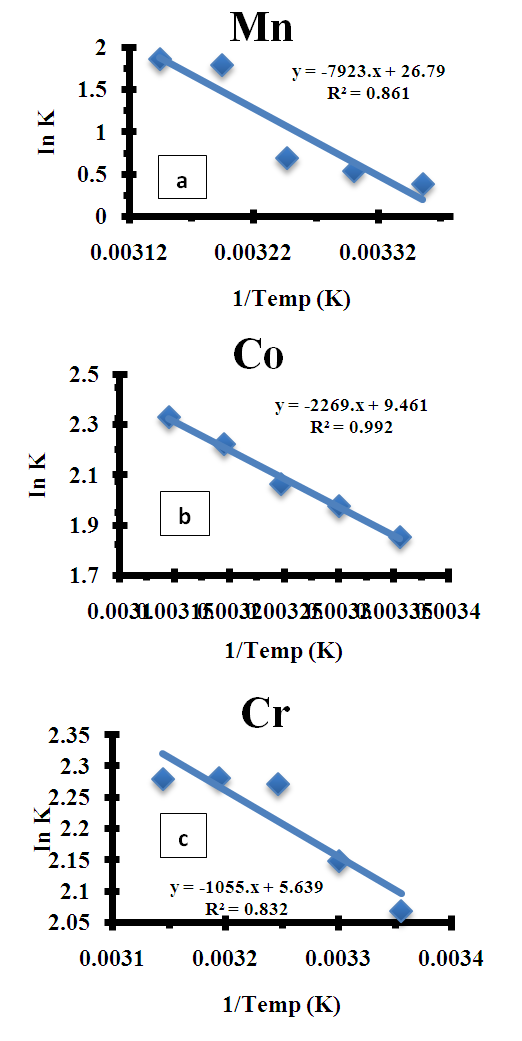 | Figure 6. Plot of Van’t Hoff for adsorption of (a) Mn2+, (b) Co2+, and (c) Cr6+onto Chrysophyllum albidum seed shell (CASS) |
4. Conclusions
- This study described the adsorption potential of Chrysophyllum albidum seed shell (CASS) as an adsorbent to efficiently remove Mn2+, Co2+ and Cr6+ ions from synthetic wastewater. The morphology of Chrysophyllum albidum seed shell before and after adsorption showed the particulate arrangement pores, macroporous structure and the roughness structure of Chrysophyllum albidum seed shell biosorbent. The FTIR spectrum revealed the presence of some functional groups appropriate for binding to enhance metal removal via adsorption. The result of the pH study showed that Chrysophyllum albidum seed shell presented different optimum adsorption performance at different pH. The highest percentage removal efficiency was observed in acidic pH range, while the optimum initial Mn2+, Co2+ and Cr6+ ion concentration for adsorption by Chrysophyllum albidum seed shell was 4 mg/L with adsorption capacities of biosorbent, increasing linearly with the initial Mn2+, Co2+ and Cr6+ concentrations. The optimum contact time required for the elimination of metal ions was 60 minutes with Chrysophyllum albidum seed shell exhibiting better selectivity towards Mn2+ adsorption, but least toward Co2+, with the trend being Mn2+ > Cr6+ > Co2+ respectively. The adsorption process was well-fitted into the Langmuir isotherm, which assumed that monolayer sorption had occurred. The kinetics data followed the pseudo-second-order model with R-squared value equivalent to 1, indicating the adsorption process was limited by chemisorption. This study aligns with the UN Sustainable Development Goal 3 (Good Health and Wellbeing) and 11 (Sustainable communities), by enhancing efficient wastewater treatment, and safeguarding human health through the removal of heavy metals from wastewater.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML