-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
International Journal of Materials and Chemistry
p-ISSN: 2166-5346 e-ISSN: 2166-5354
2024; 14(3): 31-36
doi:10.5923/j.ijmc.20241403.01
Received: Apr. 30, 2024; Accepted: May 26, 2024; Published: Jun. 7, 2024

The Effect of Mixed Surfactants on Viscosity, pH and Stability of Synthesized Liquid Soaps
Sanela Nazdrajic1, Amra Bratovcic2, Dzenita Alibegic3, Alma Micijevic4, Munir Mehovic1
1Faculty of Education, University “Dzemal Bijedic” in Mostar, Mostar, Bosnia and Herzegovina
2Department of Physical Chemistry and Electrochemistry, Faculty of Technology, University of Tuzla, Tuzla, Bosnia and Herzegovina
3Faculty of Pharmacy, University “Dzemal Bijedic” in Mostar, Mostar, Bosnia and Herzegovina
4Faculty of Agromediteranean, University “Dzemal Bijedic” in Mostar, Mostar, Bosnia and Herzegovina
Correspondence to: Sanela Nazdrajic, Faculty of Education, University “Dzemal Bijedic” in Mostar, Mostar, Bosnia and Herzegovina.
| Email: |  |
Copyright © 2024 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

This research aims to investigate the influence of mixed surfactants on the viscosity, pH, and stability of liquid soap. For this purpose, eight samples of liquid soap were synthesized. The samples were divided into two groups of four samples each. The first group of samples consisted of anionic surfactant (SLES), amphoteric surfactant (BETAIN), and non-ionic surfactant (DEA). The second set of samples consisted of the same anionic and amphoteric surfactant but a different non-ionic surfactant (APG). The following parameters were determined for all soap samples: pH value, specific gravity, viscosity, and could point. In order to examine the morphological properties, an optical microscope investigation was performed. The results show that the SLES/Betaine/DEA surfactant combination shows higher viscosity and slightly higher pH values than the SLES/Betaine/APG surfactant combination. The results also show that an increase in surfactant concentration within the system leads to an increase in viscosity as well as a slight increase in pH value. To monitor the stability of the liquid soaps obtained, the appearance, color, and odor were observed in the dark and under UV light at three different temperatures at +4°C, room temperature, and + 40°C for three months.
Keywords: Liquid soap, Surfactants, pH, Viscosity, Stability
Cite this paper: Sanela Nazdrajic, Amra Bratovcic, Dzenita Alibegic, Alma Micijevic, Munir Mehovic, The Effect of Mixed Surfactants on Viscosity, pH and Stability of Synthesized Liquid Soaps, International Journal of Materials and Chemistry, Vol. 14 No. 3, 2024, pp. 31-36. doi: 10.5923/j.ijmc.20241403.01.
Article Outline
1. Introduction
- Soap is considered the oldest cosmetic product and is significant in everyday life. The production started in the distant past, and at first, it was considered a luxury, but over time, it became a product that people cannot imagine everyday life without. The most important ingredients in cleaning products are surfactants, including laundry and household detergents, as well as personal care products. Surfactants are surface active agents that reduce surface tension and consist of a "head" (hydrophilic part) and a "tail" (hydrophobic part). [1,2] These are organic compounds with numerous applications in soaps, detergents, emulsions, paints, organic synthesis, enhanced oil recovery, lubricants, and membrane mimicry. [3]Based on the electrochemical behavior of hydrophilic groups, surfactants are divided into four categories: anionic (alkyl benzene sulfonates), cationic (quaternary ammonium salts), non-ionic (ethoxylates of fatty acids), and zwitterionic (amphoteric surfactants such as alkyl betaines). [4] The nature of the polar group largely determines the properties of surfactants and, therefore, represents one of the criteria for their classification. The largest group of surfactants are anionic surfactants, which are widely used primarily because of the simple and inexpensive production process. Anionic surfactants have a negatively charged polar group. [5] These are dissociated in water in an amphiphilic anion and a cation which is in general an alkaline metal (Na+, K+) or a quaternary ammonium. [6].The main characteristic of cationic surfactants is the positively charged hydrophilic part. The positive charge of cationic surfactants originates from the nitrogen atoms in the functional groups. Most often, it is an ammonium group or amines. [7] The use of cationic surfactants depends on their chemical structure. In detergents, they are not primary surfactants, but improve the properties of anionic surfactants.Nonionic surfactants do not ionize in water because they not have an electrical charge, which makes them resistant to water hardness deactivation. [8] They are excellent grease removers that are used in laundry products, household cleaners and hand dish washing liquids.Zwitterionic surfactants contain two functional groups of opposite charge, most often an ammonium group as a carrier of a positive charge and a carboxylate group as a carrier of a negative charge. [9] They are stable, mild and do not irritate the skin, eyes, or mucous membranes, so they are suitable for use in the cosmetic and pharmaceutical industries.Most detergent formulations contain 10 ‒ 40% of surfactants, and anionic surfactants are most often used with smaller amounts of non-ionic or amphoteric surfactants. Liquid soap usually consists of a mixture of surfactants to improve certain properties, such as cleaning performance without skin irritation. For example, sodium lauryl ether sulfate and cocamidopropyl betaine are used as surfactants to ensure foam stability and increase the viscosity of liquid soap. [10] Mixing anionic and cationic surfactants is not recommended because both lose their basic properties, e.g., anionic surfactants lose their ability to foam, and cationic surfactants lose their bactericidal effect. Also an integral part of these products are thickeners, preservatives, active ingredients, and fragrances. [11] The choice of raw materials for liquid detergent production depends on the desired properties of the finished detergent and their mutual compatibility and solubility in water. Liquid detergent formulas must ensure product effectiveness and stability regarding non-separation, sedimentation, loss or change of color, and microbiological purity. For this purpose, raw materials such as preservatives and color stabilizers are added. Preservatives protect the product from harmful microorganisms. Color stabilizers prevent color change due to the action of light or other raw materials. [12]As already mentioned, the aim of this research is to examine the influence of mixed surfactants on the viscosity, pH and stability of liquid soap. pH is essential for maintaining the skin's natural barrier. Skin cleansing is a complex interaction of skin, surfactants and water. Skin cleansing products must be mild and selective in order to remove dirt and oil from the skin without damaging the stratum corneum, the skin's main protective barrier. If the pH value of the skin is disturbed, it can become dry and flaky.Viscosity is a key parameter that determines the fluidity and dispersion characteristics of liquid soap. Viscosity is the resistance of a liquid to flow. Viscosity must be carefully controlled to achieve the desired fluidity and consistency.In order to examine the morphological properties, an optical microscope examination was performed. At lower concentrations, surfactants exist in aqueous solution in the form of monomers, while increasing the concentration of surfactants creates micelles. The concentration at which the first micelle is formed in the solution is called the critical micelle concentration. In aqueous solutions, micelles can be observed in different forms, so they can be: spherical, cylindrical, hexagonal-cylindrical and lamellar. In dilute surfactant solutions, where surfactant concentrations are not much higher than the critical micellar concentration, spherical micelles are formed. However, at higher concentrations more complex layered structures are formed, micelles can grow and above a certain concentration begin to overlap, forming a gel structure.Stability testing of liguid soap ensures that new or modified products meet physical, chemical and microbiological quality standards.
2. Materials and Methods
2.1. Materials
- The anionic surfactant used was an aqueous solution of sodium lauryl (C12-14-alkyl diglycol) ether sulfate sodium salt (Genapol® LRO, Clariant, Basel Landschaft, Switzerland), henceforth referred to as ˝SLES˝. The amphoteric surfactant used was Kapanox BC 30®, Kapachim S.A, Elton Corporation Belgrade, Serbia, henceforth, ˝Betaine˝. The two nonionic surfactants used were diethanolamine Amidet® B112, Vrbeks, Serbia, henceforth, ˝DEA˝ and C10-16 alkyl polyglycoside (Glucopon® 650 EC, BASF, Ludwigshafen, Germany), henceforth, ˝APG˝). All surfactants were used without further purification. The other chemicals used were glycerine (centrochem-Turka, Lublin, Poland), a mixture of preservatives Preventol® P100 (Lanxess, Köln, Germany) 0.1% and Salidant DMH (Salicylates & chemicals, Mumbai, India) 0.4%, dye Acid Red 131 (BASF AG, Ludwigshafen/Rhein, Germany), fragrance Pink balsam 21; Givaudan (Givaudan UK Ltd., UK), citric acid (Food chemical, Shanghai, China), sodium chloride (Solana, Tuzla, Bosnia and Herzegovina), and distilled water.
2.2. Methods
- The pH value of the liquid soap was determined using a ˝Mettler Toledo˝ pH meter in a 150 mL beaker. The pH meter electrode was inserted into the glass to measure the pH of the liquid soap solution, which is displayed on the device.The viscosity of the liquid soap was measured using a Hoppler viscometer (Sheen Instruments LTD England). The principle of this viscometer is based on dropping a glass or steel ball through a sample-filled vertical glass tube inclined at an angle close to 90° at a constant temperature. The ball has a known density and diameter, and the speed of the fall is inversely proportional to the sample's viscosity.Microscopic analysis samples were examined using reflected and transmitted light, digital optical microscope, model Motic AE310MET-T, with a color corrected optical system, widefield trinocular 45°, 280x180mm surface - 50x50mm movement, magnification 160x.
2.3. Preparation of Soap Solutions
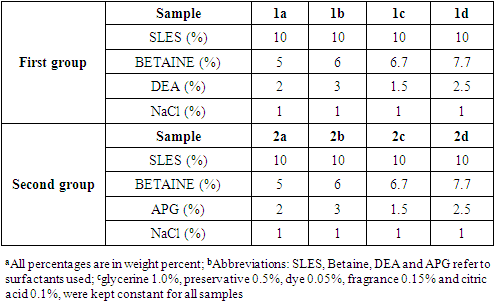
- Two groups of liquid soap samples were created. Each group had four different samples (Table 1). All soap solutions contained 10% SLES. All samples in the set shared the same amphoteric (betaine) surfactant concentrations but different nonionic surfactant components (DEA for the first and APG for the second group).The solution was prepared by dissolving 600 grams of SLES in water at 38°C. After mixing for 20 minutes, other surfactants were added and mixed for another 10 minutes until the solution was clear and homogeneous. Glycerin was then added, increasing the turbidity of the solution. A citric acid solution was added to adjust the pH. After adjusting the pH, preservatives, dye, and fragrance were added, and the solution was stirred until it became clear and homogenous. Finally, 1 wt% sodium chloride was added slowly to regulate the viscosity of the solution.
|
3. Results and Discussion
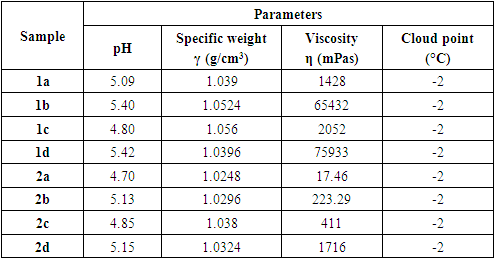
- The following parameters were evaluated for each soap sample: pH value, specific gravity,viscosity, and cloud point. The results are shown in Table 2.
|
3.1. Effect of Mixed Surfactants on pH Value of Liquid Soap
- One of the requirements for the quality of liquid soap is the pH value. That is because liquid soap is in direct contact with the skin and can cause problems if its pH does not match the pH of the skin. Personal care products and cosmetics with a pH that is too high or too low can cause skin irritation by increasing the skin's absorbency. To prevent this, the pH of such products must be similar to that of the skin, which typically ranges between 4.5 and 7.0. [13] Figures 1 and 2 show the pH values of eight different prepared samples of liquid soaps.
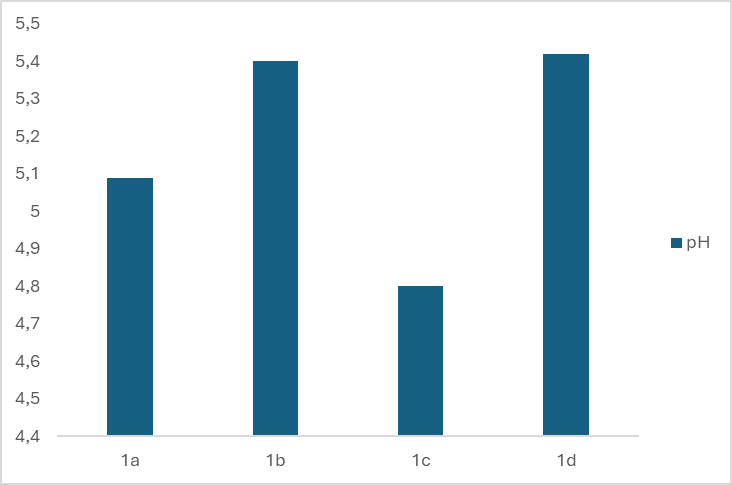 | Figure 1. pH of four different types of liquid soap in the first group |
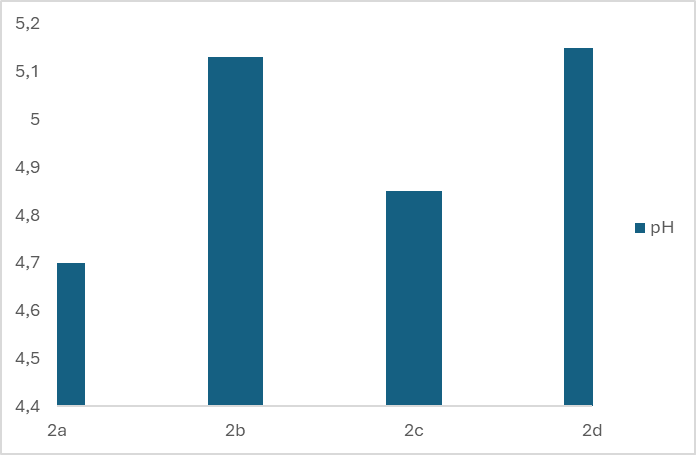 | Figure 2. pH of four different types of liquid soap in the second group |
3.2. Effect of Mixed Surfactants on Specific Weight
- A study was conducted to investigate how the specific gravity of liquid soap is affected by the type and ratio of surfactants used in its formulation. One of the factors that affects the change in the specific gravity of the solution is the type and concentration of the dissolved material in it. Figure 3 shows the specific weight of eight different prepared samples of liquid soaps.
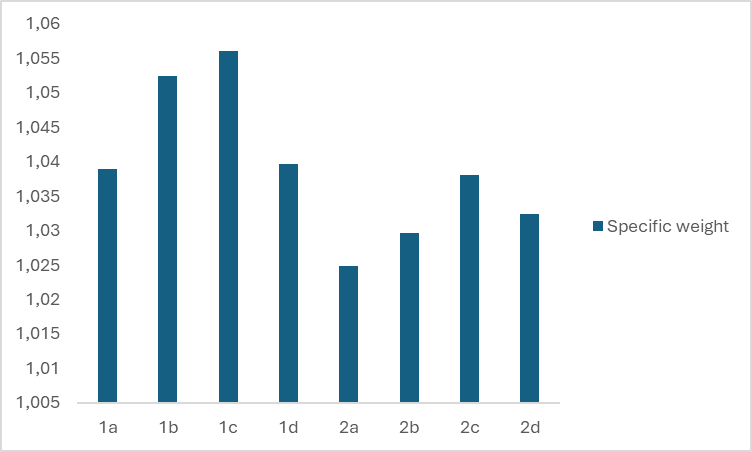 | Figure 3. Specific weight of eight different types of liquid soap |
3.3. Effect of Mixed Surfactants on Viscosity
- Viscosity is one of the physical properties of liquid soap that can be used as a product quality parameter. Viscosity is important both for stability and use of cosmetic products.The viscosity of shampoos and liquid soaps is between 400 and 4000 mPas. [16] Figures 4 and 5 show the viscosity, η (mPas), of eight different types of liquid soaps. Figure 4 shows that the concentration increase of the amphoteric surfactant Betaine and nonionic DEA by 1% in the first group of liquid soap leads to a sudden increase in viscosity from 1428 to 65432 mPas. Adding the surfactants Betaine and DEA to substances containing the anionic surfactant SLES increases the viscosity of the formulation, which is higher than the commercially desirable value. A continuous increase in surfactant concentration causes a large increase in viscosity. By increasing the concentration of amphoteric surfactant by 2.7% and nonionic surfactant by 0.5%, the viscosity increases from 1428 to 75933 mPas. Increasing the proportion of amphoteric surfactant by 1.7% while reducing the concentration of nonionic surfactant by 0.5% also leads to an increase in viscosity, but not as sharply as in sample 1b, and the measured value for sample 1c is 2052 mPas.
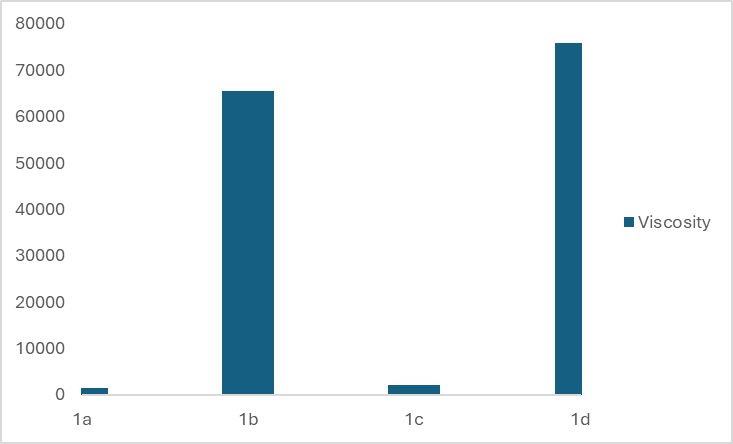 | Figure 4. Viscosity, η (mPas), of four different types of liquid soap in the first group |
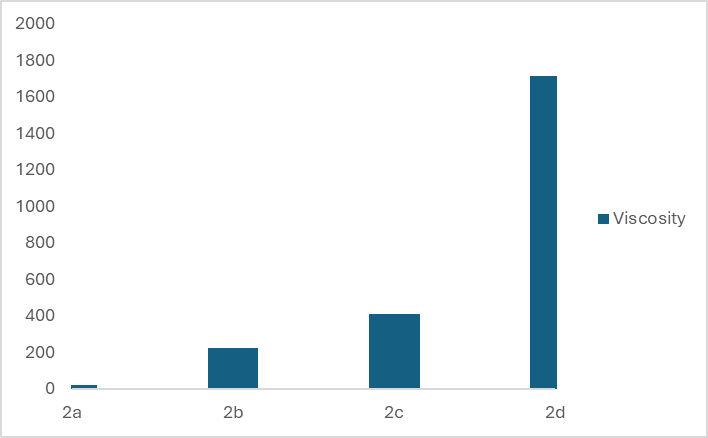 | Figure 5. Viscosity, η (mPas), of four different types of liquid soap in the second group |
3.4. Stability of Liquid Soaps
- Personal hygiene products are subject to a natural degradation process over time, commonly referred to as the product's shelf life. The expiration date is the manufacturer's guarantee that the product will be usable, efficient, and have the prescribed organoleptic properties following the manufacturer's specification. Evaluation of liquid soap products can be seen organoleptically, among other things, in terms of appearance, smell, and color. The sensory analysis procedure obtained data on the stability of synthesized liquid soaps. All synthesized liquid soaps were stored in plastic containers and the appearance, color, and fragrance were monitored at three different temperatures, +4°C, room temperature, and +40°C, but also in the dark and under the influence of UV light for three months. In all samples of the first and second groups of liquid soap, no change was recorded at the tested temperatures. All samples were stored at a temperature of -2°C, and no turbidity occurred in any liquid soap samples, as shown in Table 1. It is known that if a product passes this type of test, it is a good indicator of the long-term stability of the product.
3.5. Morphological Analysis
- The surfactant mixture forms a monomolecular layer on the surface and mixed micelles inside the solution (see Figures 6 and 7).The pictures presented illustrate the formation of mixed micelles that occur when anionic and nonionic surfactants are mixed. Since the synthesized liquid soap is a complex multiphase system that contains several emulsifiers - anionic/nonionic/amphoteric surfactants together they form the so-called mixed surfactants.The forming of a lamellar phase (liquid-crystalline and gel) that incorporates large amounts of water is responsible for the stability of emulsions made using these emulsifiers. An excellent example is Figure 6, samples a, b, c, and d, which are gel networks, and the difference in surfactant content leads to a smaller or larger amount of water between the micelles, seen as thinner and thicker micelles.
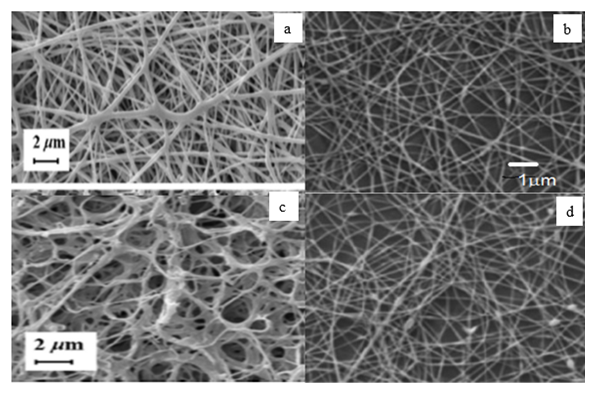 | Figure 6. Morphology of liquid soaps: a) sample 1a; b) sample 1b; c) sample 1c; d) sample 1d |
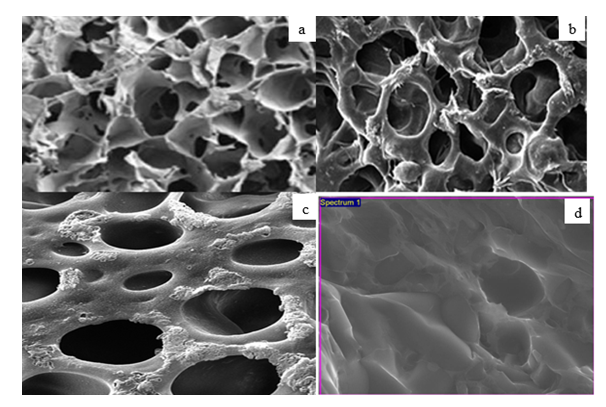 | Figure 7. Morphology of liquid soaps: a) sample 2a; b) sample 2b; c) sample 2c; d) sample 2d |
4. Conclusions
- For this research, liquid soaps were prepared from anionic, nonionic, and amphoteric surfactants containing sodium chloride, glycerin, preservative, color, fragrance, citric acid, and water. Research results showed that the difference in the concentration of surfactants used affects the characteristics of the produced liquid soap.In synthesized liquid soaps, the pH ranged from 4.70 to 5.42. Replacing the nonionic surfactant DEA with the nonionic surfactant APG in the second group of samples led to a slight change in the pH value, but all samples remained slightly acidic. Based on the results for the specific gravity, it can be concluded that the interaction of the surfactants used has no significant influence on the value of the specific gravity of the synthesized liquid soap.The results of the effect of the type and ratio of surfactants on the viscosity of liquid soap show that the combination of surfactants SLES/Beatin/DEA in the ratio 10/5/2 as well as in the ratio 10/6.7/1.5, i.e., samples 1a and 1c, produce soaps with a viscosity that is acceptable to consumers. Samples of liquid soaps 1b and 1d have a high viscosity that considerably exceeds the recommended values. Research has shown that using nonionic surfactant APG instead of nonionic surfactant DEA in system 1 results in much lower viscosity values. Sample 2d, the combination of surfactants SLES/BETAIN/APG in the ratio 10/7.7/2.5, exhibits a viscosity of 1716 mPas, which is the most acceptable measured viscosity of the second group. Morphological images of liquid soaps indicate the formation of mixed micelles through hydrophobic interactions. Several examples of micelle formation and lamellar phase formation are presented. The parameters that initiate the creation of such structures are primarily the change in the concentration of individual surfactants, as well as the concentration ratio of their mixtures and then the geometry of the molecules. All of the samples tested were found to be stable, and no turbidity was observed in any of them.
ACKNOWLEDGEMENTS
- The authors gratefully acknowledge the DITA 1977 detergent industry in Tuzla, Bosnia and Herzegovina which has given us the support in using their equipment and chemicals for research.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML