Bibigul Koshanova Turganbaevna, Dildora Tursunova Abdusattarovna, Aktam Erkaev Ulashovich, Nazokat Erkaeva Aktamovna
Department of Chemical Technologies of Inorganic Substances, Tashkent Chemical Technology Institute, Tashkent City, Uzbekistan
Correspondence to: Bibigul Koshanova Turganbaevna, Department of Chemical Technologies of Inorganic Substances, Tashkent Chemical Technology Institute, Tashkent City, Uzbekistan.
| Email: |  |
Copyright © 2024 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Abstract
The aim of the work is to study the theoretical foundations for obtaining Na2SO4·0.5H2O2 by liquid-phase and solid-phase methods and to study its bleaching properties. In order to obtain bleaches, a theoretical analysis of the systems: sodium sulfate - hydrogen peroxide - water and potassium carbonate - hydrogen peroxide - water was carried out. Based on the chemical analysis of the compositions of liquid and solid phases, isothermal diagrams of the solubility of these systems at 10 and 20°C were drawn. In order to obtain synthetic detergents and theoretical analyzes of the literature data, the mutual solubility of salts in the ternary system sodium sulfate - hydrogen peroxide - water was studied by the isothermal method. The obtained data of chemical analysis were used to determine the compositions of solid phases according to Schreinemakers method and drawing diagrams of solubility at 25°C. The solid-phase process of treating sodium sulfate (thenardite) powder with hydrogen peroxide at various rates was also studied. The liquidus curve of the solubility diagram for the sodium sulfate-peroxide-hydrogen-water system is characterized. The mineralogical composition of the products obtained by the liquid-phase and solid-phase methods was determined by X-ray phase, thermal catalytic and electron microscopic methods of analysis. X-ray phase analysis was used to determine the mineralogical composition of the products, thermal analysis was used to determine the thermal stability of the obtained samples, and electron microscopic analysis was used to determine the particle sizes and their compositions. The physics - mechanical properties of the obtained products are determined, which are the initial moisture content, apparent density, density with compaction, angle of inclination and hygroscopic point.
Keywords:
Sodium sulfate, Hydrogen peroxide, Sodium peroxysulfate, System, Synthetic detergent, Surface active agent, Ratio and crystal
Cite this paper: Bibigul Koshanova Turganbaevna, Dildora Tursunova Abdusattarovna, Aktam Erkaev Ulashovich, Nazokat Erkaeva Aktamovna, Development of Technology for Obtaining Sodium Peroxide Sulfate, International Journal of Materials and Chemistry, Vol. 14 No. 2, 2024, pp. 24-29. doi: 10.5923/j.ijmc.20241402.03.
1. Introduction
Currently, wide ranges of synthetic detergents are produced in the world, but they consist mainly of tripolyphosphate - a substance that does not wash itself, but only prepares water for washing. Washes the rest - surfactants, bleaches, enzymes and other ingredients. Tripolyphosphate is the cheapest, but also the most dangerous of them for human health and the environment. In many countries, the use of tripolyphosphate in synthetic detergents is prohibited. In this regard, the creation of cheap and environmentally friendly synthetic detergents from local materials is an urgent problem.In recent years, among household chemical goods, one of the dynamically developing, constantly increasing amount of production is a group of bleaching agents. Modern bleaching agents should be considered as independent agents for bleaching linen, and as auxiliary agents for processing linen during the washing process. These goals are achieved with optical and chemical bleaches. The bleaching effect is also achieved chemically. Oxidative bleaching has gained practical importance. Currently, it is carried out using peroxides and hypochlorites. The essence of bleaching lies in the fact that active oxygen radicals interact with the chromophore parts of pollutant molecules, turning them into uncolored compounds or white compounds.The source of hydrogen peroxide is its aqueous 30% solution. Hydrogen peroxide is currently one of the most important oxidizing agents widely used in chemistry and chemical technology. Its main advantage is ecological compatibility; the main disadvantage is the inconvenience during transportation due to the high (60-70%) water content. In this regard, along with a solution of hydrogen peroxide, solid peroxides are also used - sodium perborate, sodium percarbonate, urea peroxide and others [1-5].
2. Materials and Methods
Based on this, in order to obtain synthetic detergent and theoretical analyzes of the literature data [6-9], we studied the mutual solubility of salts in the ternary system sodium sulfate - hydrogen peroxide - water was studied by the isothermal method [10-12] at 20°C (table and figure). Chemical analysis of liquid and solid phases was carried out by known methods of analytical chemistry. The data obtained were used to determine the compositions of the solid phases according to Schreinemakers and drawing diagrams of solubility at 25°C.The phase equilibrium in the system was established with continuous mixing and thermostated after 0.5 and 1 days, respectively. Based on the chemical analysis of the compositions of the liquid and solid phases, an isothermal diagram of solubility of this system at 25°C is formed. In the sodium sulfate system - hydrogen peroxide - water at 20°C is the formation of a new compound of the composition Na2SO4 ∙ 0.5H2O2. The liquids curve of the solubility diagram of the sodium sulfate system - hydrogen peroxide - water at the studied temperature is characterized by the presence of three branches of crystallization of the original components and the new compound of the composition - Na2SO4∙0.5H2O2. The connection formed in the studied system is identified by the chemical method. To obtain the compound, a solution of the composition figurative point N was prepared: Na2SO4-40.0%, H2O2-30.0% and H2O-30% which was stirred at 35°C for 10 minutes and cooled to 25°C. The formed solid phase from the suspension was separated by filtering, washed, and dried at 60°C.
3. Results and Discussion
Chemical analysis of the solid phase, selected from the alleged crystallization of the NA2SO4∙0.5H2O2 compound, gave the following results: defined, %: Na – 30,42; O2 –5,90. For Na2SO4∙0.5H2O2, it is calculated, %: Nа –30,46; O2 – 5,96.Table 1. Nodular points of the isotherm of the system Na2SO4 ∙ - H2O2 - H2O at a temperature of 25°C
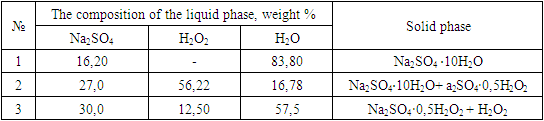 |
| |
|
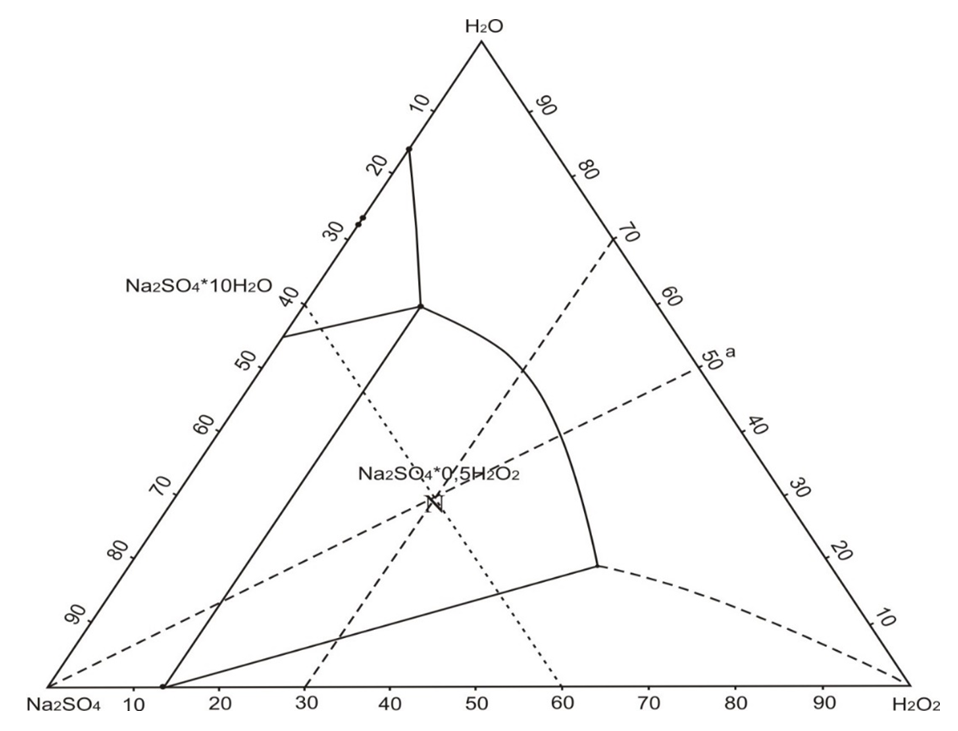 | Figure 1. Isothermal diagram of system solubility Na2SO4-H2O2-H2O at 25°C |
It was also studied the process of a solid-phase method for obtaining sodium sulfate peroxide by processing sodium sulfate (thenardite) powder with hydrogen peroxide at a rate of 80, 100 and 110%. The data obtained are shown in Table 2.Table 2. Influence of technological processes on the quality of peroxysulfate
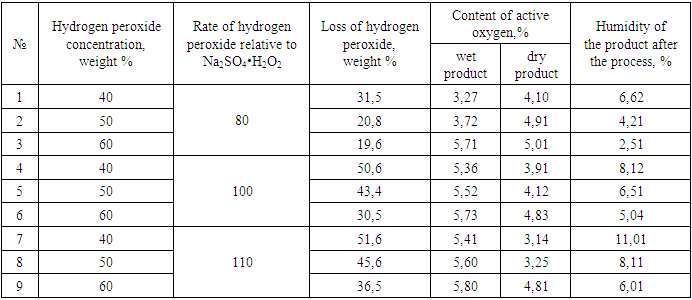 |
| |
|
Table 2 shows that the content of active oxygen in wet and dry products ranges from 3.27-5.73 and 3.12-5.01%, respectively. The moisture content of the product does not exceed 2.51-6.62% at a hydrogen peroxide rate of 80% in the range of the latter's concentration of 40-60%. With an increase in the rate of hydrogen peroxide from 100% to 110%, the ranges of humidity fluctuations increase and amount to 5.04-8.12% and 6.011-11.01%, respectively. In a dry product, with an increase in the rate of hydrogen peroxide, the content of active oxygen in products decreases.This is explained by the fact that with an increase in the rate of hydrogen peroxide, the moisture content of the products increases and the content of hydrogen peroxide in the liquid phase increases proportionally, which is easily released from the product during drying. Accordingly, with an increase in the norm from 80.90 to 110%, the loss of hydrogen peroxide increases from 14.6, 20.8 and 31.5 to 35.5, 78.6 and 51.6%, respectively, at a hydrogen peroxide concentration of 40% and 60%.Based on the foregoing, it can be concluded that with a decrease in the concentration and an increase in the rate of hydrogen peroxide, peroxide losses increase and the product yield relative to hydrogen peroxide is reached more than 80% at a rate of 80% use of hydrogen peroxide with a concentration of at least 50%.To determine the mineralogical composition of the samples, X-ray phase analysis was used [13-15] (Fig. 2 and Table 3), which showed that the mineralogical composition of the obtained product consists of Na2SO4·0.5H2O2, Na2SO4·10H2О and Na2SO4. At temperatures above 35°C mirabilite completely transforms into thenardite after a certain period of time. The main constituent mineral of the samples is Na2SO4·0.5H2О2, the content of which is more than 80%.Table 3. Mineralogical composition of the reaction products according to X-ray phase analysis
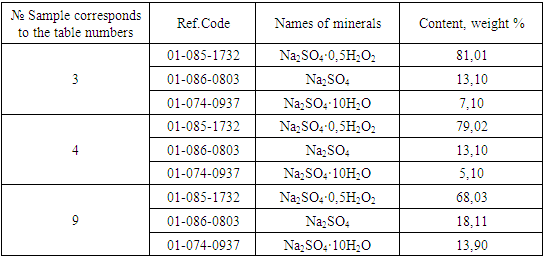 |
| |
|
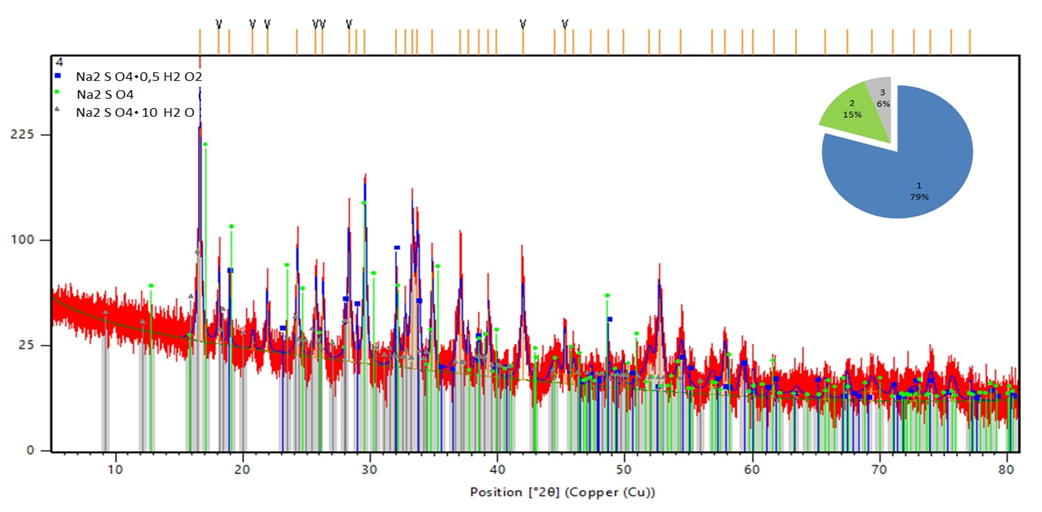 | Figure 2. X-ray pattern of samples |
Morphological studies of the surface of the samples were carried out using a scanning electron microscope SEM - EVO MA 10 (Zeiss, Germany). For the experiment, on a round holder made of a metal alloy, on top of which an aluminum foil with a double-sided adhesive surface was glued, which sample powders were applied. This experiment is to study the microstructure of the surface, as well as to determine the size of microparticles. During the measurement, an accelerating voltage (EHT - Extra High Tension) of 20.00 kV was applied; the working distance (WD-working distance) was 8.5 mm. The measurement was carried out in the mode of detecting secondary electrons (SE1-secondary electrons detector). The images were acquired at various scales using the Smart SEM software. The results of the study are illustrated in fig.3.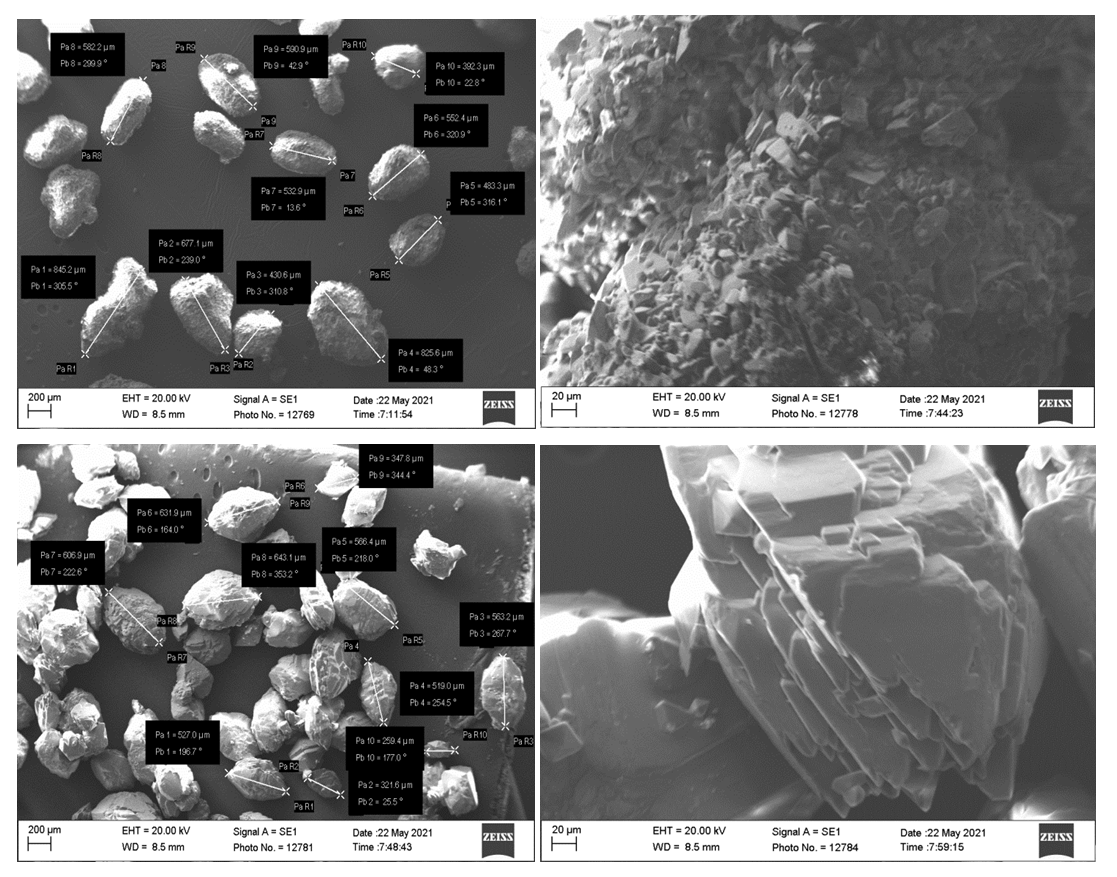 | Figure 3. Microscopic images of samples 3.9. Sample numbers correspond to those in table 2 |
Figure 3 clearly shows the main particles with an average size of 572 and 495.5 mm, covered with minerals of sodium peroxide sulfate Na2SO4·0.5H2O2, and inside the particles there is thenardite. When using 60% hydrogen peroxide, plate-shaped Na2SO4·0.5H2O2 particles with dimensions of 5-15 mm are formed (Fig.3. sample.3 b), as well as mirabilite particles with dimensions of 100x80x300 mm., (Fig.3. sample. 9 d) [16].To determine the thermal stability of the obtained samples, thermal analytical studies were carried out on a Netzsch Simultaneous Analyzer STA 409 PG instrument. Sample holders TG, TG-DTA are equipped with a thermocouple for direct temperature measurement in the sample/reference crucible. Temperature range: 25-3700C. Thermoanalytical studies of samples in the amount of 5-10 mg were carried out in an inert nitrogen atmosphere with a nitrogen flow rate of 50 ml/min [17-20].Figure 4 shows that the decomposition of the product starts at a temperature of 60.1°C and continues to a temperature of 133.1°C, and the total mass loss is 6.0-7.0%.Figure 4 shows that the decomposition of the product begins at a temperature of 60.1°C and continues to a temperature of 133.1°C, and the total weight loss is 6.0-7.0%.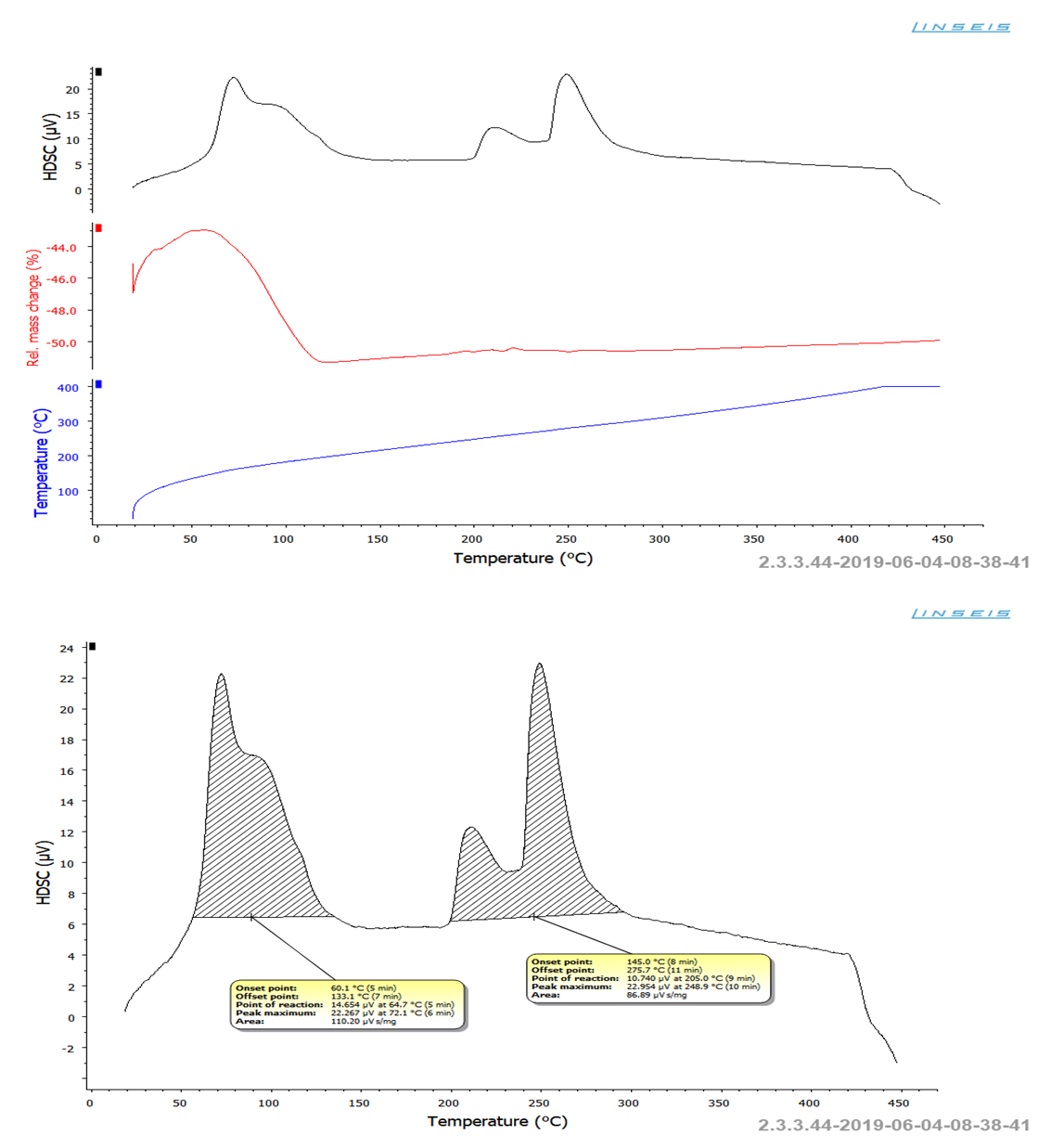 | Figure 4. Derivatogram of products (numbers of samples correspond to numbers in tab. 2, sample 9) |
This process on the HDSC curve looks like a doublet maximum characterizing the decomposition of mirabilite and sodium sulfate peroxide with the release of water and elemental oxygen. Also on the HDSC curve, a second doublet maximum is observed in the temperature range 145-275.7°C, which is characteristic of the phase transformation of sodium sulfate without weight loss.
4. Conclusions
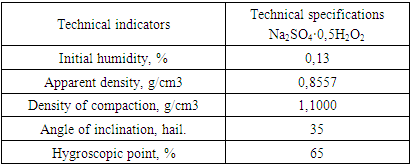
The physical and mechanical properties of the obtained product are also determined (Table 4). The data obtained show that the hygroscopic point of the product is 65%, i.e. it corresponds to low-hygroscopic substances.Table 4. Physical and chemical properties of Na2SO4·0.5H2O2
 |
| |
|
Thus, the conducted studies show that Na2SO4·0.5H2O2 develops good bleaching properties and can be obtained in two ways: liquid-phase and solid-phase.
References
| [1] | Erkayeva N.A., Allanazarova Z.А., Kucharov B.Х., Kaipbergenov А.T., Erkayev А.U. 2015. Preparation of chemical bleaches based on potassium carbonate, sodium sulfate and hydrogen peroxy, Proceedings of the Respublica scientific theoretical and scientific conference on the topic “Best practices in science and education: confirmation and results” dedicated to the year of honoring the elderly p. 342-344. |
| [2] | Wada T., Koga N. 2013 Chemical Composition of Sodium Percarbonate: An Inquiry-Based Laboratory Exercise J. Chem. Educ. 90 1048-1052. |
| [3] | Pritchard R.G., Islam E. 2003. Sodium percarbonate between 293 and 100 K Acta Crystallorg., Sect. B: Struct. Sci. 59 596-605. |
| [4] | Zhao H.K., Wang H.X., Du M.J. 2002. Research on Thermal Decomposition Kinetics of Sodium Percarbonate Shangqiu Shifan Xueyuan Xuebao 18 79-81. |
| [5] | Wang H.X., Zhao H.K., Du M.J., Bai Y.J. 2003. Research on Thermal Kinetics of Sodium Percarbonate Shangqiu Shifan Xueyuan Xuebao 19 94-97. |
| [6] | Zadanovsky A.B., Lyakhovskaya E.I., Shleymovich R.E. 1953. Handbook of experimental data on the solubility of multicomponent water-salt systems. Volume 1. Ed. "Chemistry", M.: 337 p. |
| [7] | Handbook of solubility. In 3 volumes / Kogan V.B., Ogorodnikov S.K., Kafarov V.V., under. ed. Kafarova V.V. 1969. - L.: Science. T. 3. Book. 2. - 1170 p. |
| [8] | Measurement technique (algorithm) 2016. Determination of caking of mineral fertilizers. N. 1104-00209438-146-2016. JSC "RIFIF". |
| [9] | Ngo T.D. et al. 2018. Additive manufacturing (3D printing): A review of materials, methods, applications and challenges. Composites Part B: Engineering 143 172-196. |
| [10] | Van't Hoff Ya. G. Oceanic salt deposits. - L.: ONTI Himteoret. b 1936. - 344 p. |
| [11] | Budaragin AN 2006 Decisions «Dow Corning» Household chemistry 23 8. |
| [12] | Kotomin A.A., Yakimchuk O.D. 2005. Investigation of the detergent action of SMS compositions Household Chemistry 20-23. |
| [13] | Doebelin N., Kleeberg R., 2015. “Profex: a graphical user interface for the Rietveld refinement program BGMN”, Journal of applied crystallography, no. 48(5), pp.1573-1580. |
| [14] | Mayo R.A., Marczenko K.M., Johnson E.R. 2023. Quantitative matching of crystal structures to experimental powder diffractograms. Chem Sci. Apr 4; 14(18): 4777-4785. doi: 10.1039/d3sc00168g. PMID: 37181772; PMCID: PMC10171065. |
| [15] | Sheldrick G.M. 2015 Jan 1. Crystal structure refinement with SHELXL. Acta Crystallogr C Struct Chem. 2015 Jan; 71(Pt 1): 3-8. doi: 10.1107/S2053229614024218. Epub PMID: 25567568; PMCID: PMC4294323. |
| [16] | Patrick Echlin. 2009. Handbook of Sample Preparation for Scanning Electron Microscopy and X-Ray Microanalysis, Cambridge Analytical Microscopy, UK, Springer. -330p. |
| [17] | José M. Fernández, César Plaza, Alfredo Polo, Alain F. Plante. 2012. Use of thermal analysis techniques (TG – DSC) for the characterization of diverse organic municipal waste streams to predict biological stability prior to land application. January -Pages 158-164. |
| [18] | Jing Guo, Han Zhi, Liu Gun. Investigation on Thermal Decomposition Kinetics of Na2SО4·0.5H2О2·H2О / Journal of Zhengzhou University (Engineering Science) Vol-28 № 3. 2007. |
| [19] | Ito Y., Mashiko T. 1977. Stable sodium sulfate-hydro- hen peroxide-sodium chloride adduct and process for preparing same [P] US: 4005182. |
| [20] | Barbara Charmas, Karolina Kucio, Volodymyr Sydorchuk, Svitlana Khalameida, Magdalena Ziezio and Aldona Nowicka. Characterization of Multimodal Silicas Using TG / DTG / DTA, Q-TG, and DSC Methods. Faculty of Chemistry, Department of Chromatographic Methods, Maria Curie-Skłodowska University, Maria Curie-Skłodowska Sq. 3, 20-031 Lublin, Poland. |







 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML


