-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
International Journal of Materials and Chemistry
p-ISSN: 2166-5346 e-ISSN: 2166-5354
2024; 14(2): 19-23
doi:10.5923/j.ijmc.20241402.02
Received: Feb. 9, 2024; Accepted: Mar. 13, 2024; Published: Mar. 29, 2024

Determination of Zinc Ion by Inversion Voltammetric Method from the Composition of Wastewater Using an Electrochemical Sensor
Maqsud N. Sayfiyev1, Maqsuda A. Nazarova2, Qudrat S. Boqiyev2, Abdushukur A. Gofurov2, Dilshod A. Ziyayev3
1Teacher of the National University of Uzbekistan named after Mirzo Ulugbek
2PhD Student of the National University of Uzbekistan named after Mirzo Ulugbek
3Associate Professor of the National University of Uzbekistan named after Mirzo Ulugbek
Correspondence to: Maqsud N. Sayfiyev, Teacher of the National University of Uzbekistan named after Mirzo Ulugbek.
| Email: |  |
Copyright © 2024 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

In this study, advanced techniques for the precise detection of zinc ions in minute quantities within wastewater samples have been innovatively developed through the customization of electrochemical sensors using the inversion-voltammetric method. This research not only presents the novel methodologies established for the efficient identification of zinc ions but also focuses on enhancing the metrological characteristics associated with zinc ion determination through the inversion-voltammetric approach. By refining the assessment protocols and methodologies, this study contributes significantly to the field of analytical chemistry, paving the way for more accurate and reliable monitoring of zinc ion levels in wastewater, thereby facilitating improved environmental management practices and ensuring the protection of public health.
Keywords: Microelement, Wastewater, Zinc, Background electrolyte, Stripping voltammetry, Adjacent cations, Electrochemical sensor, Trace element, Side cations, Buffer mixture
Cite this paper: Maqsud N. Sayfiyev, Maqsuda A. Nazarova, Qudrat S. Boqiyev, Abdushukur A. Gofurov, Dilshod A. Ziyayev, Determination of Zinc Ion by Inversion Voltammetric Method from the Composition of Wastewater Using an Electrochemical Sensor, International Journal of Materials and Chemistry, Vol. 14 No. 2, 2024, pp. 19-23. doi: 10.5923/j.ijmc.20241402.02.
1. Introduction
- Today, the relentless growth in people's demand for industry, economy, food, and various products is a prevailing trend, exacerbating the volume of wastes discharged into the environment, notably wastewater [1-2]. The detrimental impact on flora and fauna stemming from these environmental emissions is intensifying at an alarming rate, underscoring the pressing need for contemporary analysts to devise increasingly precise and effective methodologies. This imperative arises from several critical factors: firstly, the imperative to oversee and regulate product quality to align with contemporary standards; secondly, the essential task of averting environmental degradation caused by waste products generated in the production processes; and thirdly, the urgent issue of escalating levels of heavy metals in wastewater, posing a direct threat to human health.The surge in heavy metal concentrations within wastewater presents a multifaceted challenge, necessitating immediate attention to mitigate its adverse consequences on both environmental ecosystems and public well-being. Of particular concern is the link between industrial and economic activities and the contamination of wastewater with heavy metals, which in turn contributes to the proliferation of severe illnesses and health hazards among populations exposed to such pollutants.In essence, the escalating demands of our modern society underscore the critical importance of implementing innovative strategies and sustainable practices to curb environmental degradation and foster a more harmonious coexistence between human activities and the natural world. By promoting responsible resource management, fostering interdisciplinary collaboration, and championing advancements in environmental stewardship, we can strive towards a cleaner, healthier, and more sustainable future for all.Considering the formidable challenges outlined above, the focal point of this research endeavor is dedicated to the meticulous analysis and extraction of zinc ions, a notorious constituent known for its high carcinogenicity, from wastewater compositions. By delving into the intricate complexities of isolating and quantifying this hazardous element, this study aims to shed light on the critical importance of mitigating the presence of zinc ions in wastewater to safeguard environmental integrity and public health.
2. Materials and Methods
- The experimental data obtained from the analysis were meticulously documented employing a sophisticated setup comprising a graphite-based electrochemical sensor, a reference electrode saturated with potassium chloride, and an electrolyzer featuring an auxiliary graphite electrode with an expansive surface area. Furthermore, the research apparatus included the utilization of an ABC-1.1 device integrated with a computer system for data processing and analysis. Noteworthy instrumentation incorporated in the study encompassed an ACZET PVT LTD CY 224C analytical balance, a high-precision Swiss-made pH meter model pH/Mv/TEMP m FiveEasy F20, and a cutting-edge magnetic stirrer model MS-H280-Pro, each playing a pivotal role in ensuring the accuracy and reliability of the experimental procedures [3].
3. Results and Discussion
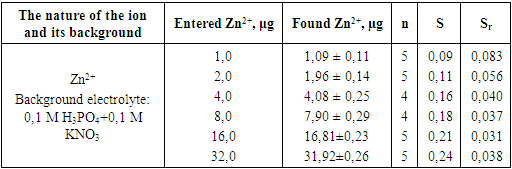
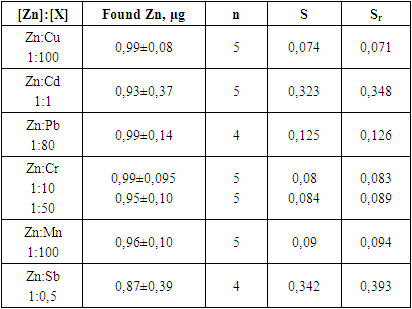
- We started this research work by first preparing our working device and electrochemical sensor. For this:1. Preparing the voltage analyzer for work.The analyzer, the electrochemical sensor "EM-04 module" and the personal computer should not be installed on a table in a room with minimal vibrations from sources of strong electromagnetic fields (power transformers, electric motors, electric furnaces).The "EM-04 module" is connected to the microprocessor unit via a cable to the corresponding connector on the back panel of the ABC - 1.1 voltammetric analyzer.Connect the analyzer and the computer to the power source.2. Preparation of electrodes for use.2.1. Preparation of EVL - 1M4 reference electrode.Before use, the electrode should be prepared as follows:• The rubber ring is moved down;• The electrode cavity is washed with bidistilled water;• Using a pipette from the filling hole, the electrode space is filled with a saturated solution of potassium chloride;• The electrode is left in a saturated solution of potassium chloride for 48 hours;2.2. Preparation of the measuring (working) electrode.Before using the working electrode (ES), the working surface of the measuring electrode must be wiped with ethyl alcohol and washed with bidistilled water, which prevents moisture from entering the threaded hole.In our further work, we chose favorable conditions for zinc ion determination by inversion voltammetric (IV) ES method. First of all, the influence of the background electrolyte in the determination of zinc ion by the IV method was studied.Control of the electrochemical reaction requires control of the role of the background electrolyte and buffer mixture in the proton-donating activity of the medium and the concentration of the detectable ion within strictly defined limits throughout the electrolysis. During the experiments, the following solutions are used as background electrolytes: 0,1 M H3PO4; 0,2 M HCl; 1,0 M KSCN; 0,1 M H3PO4 +0,1 M KNO3; 1,0 M KCl + 0,2 M HNO3; 1,0 M LiCl; 1,0 M KNO3 va 1,0 M NaNO3 + 1,0 M HF. We used different volumes and concentrations of these background electrolytes and buffer mixtures [4].Before selecting the background electrolyte, zinc ion was determined in the presence of ordinary bidistilled water. First, we took 1.0 ml of the standard solution (10,000 μg/dm3) containing zinc ions to be determined, put it in the electrochemical cell, and added 24 ml of bidistilled water. No analytical signal was observed when the resulting solution was analyzed. The reason is the low electrical conductivity of the solution, as well as the low activity of metal ions detected in the solution. In such cases, the use of background electrolyte solutions is appropriate and ensures the reliability of the analysis. The obtained results are presented in Figure 1.
 | Figure 1. Graph of determination of zinc ion from standard solution without background electrolyte |
 | Figure 2. Effect of background electrolyte in the determination of zinc ion from a standard solution |
|
|
 | Figure 3. Graph of the dependence of the analytical signal on the concentration of zinc ions in the determination of zinc ions by the inversion voltammetric method |
|
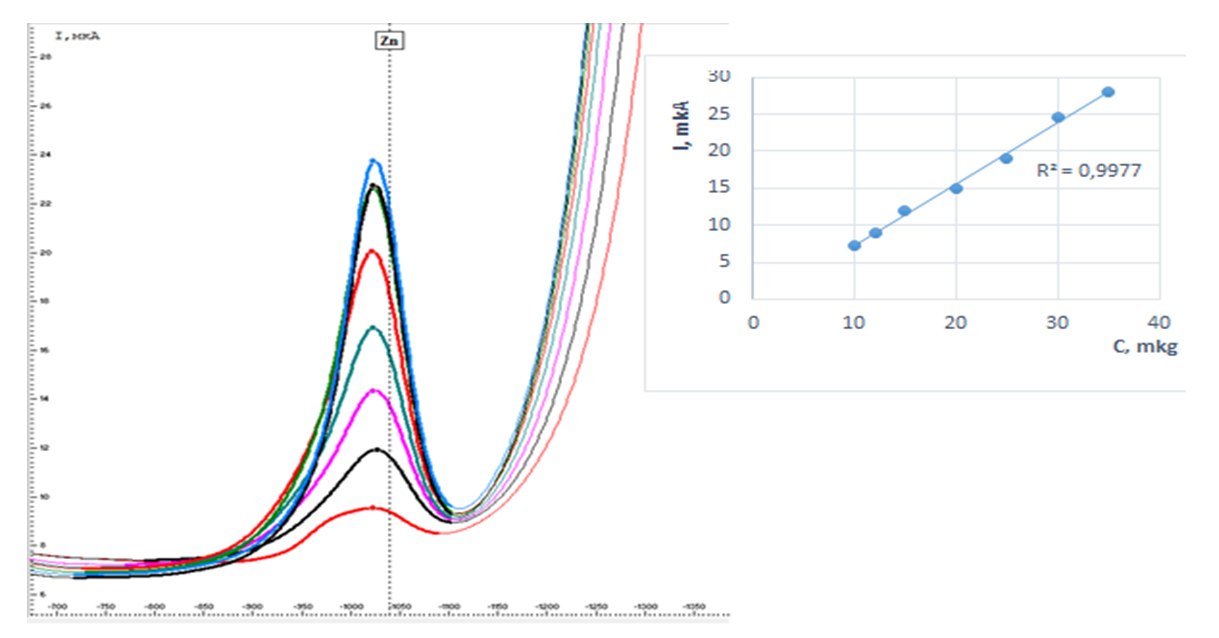 | Figure 4. Graph of the dependence of the analytical signal on the concentration of zinc ions in the determination of zinc ions by the inversion-voltammetric method |
|
|
4. Conclusions
- In the determination of zinc ion by inversion voltammetric method, favorable conditions were selected (pH value of inversion buffer mixture as background electrolyte is 5.6, and 0,1 M H3PO4+0,1 M KNO3, accumulation time on the electrode surface is 120 seconds).Using the developed method, zinc ion was determined from individual, binary and model artificial mixtures. The obtained results showed that it is possible to determine zinc ion from individual, binary and model artificial mixtures with the help of our developed method, and the relative standard deviation did not exceed 0.083.Using the created inversion voltammetric method in the analysis of model binary, ternary and complex mixtures, it was possible to draw a conclusion from the results obtained in the inversion voltammetric determination of the studied metal, according to which the developed methods are similar in nature and concentration to real natural objects and showed that it can be used in soil.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML