-
Paper Information
- Next Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
International Journal of Materials and Chemistry
p-ISSN: 2166-5346 e-ISSN: 2166-5354
2023; 13(2): 23-27
doi:10.5923/j.ijmc.20231302.02
Received: Sep. 11, 2023; Accepted: Sep. 26, 2023; Published: Oct. 13, 2023

Solubility of Components in the (NH4)2HPO4-NH(C2H4OH)2-H2O System
Saidolim Mardanov1, Shokhida Khamdamova2
1PhD Student, Fergana Polytechnic Institute, Fergana, Uzbekistan
2Doctor of Sciences (DSc), Professor, International Institute of Food Technology and Engineering, Fergana, Uzbekistan
Correspondence to: Saidolim Mardanov, PhD Student, Fergana Polytechnic Institute, Fergana, Uzbekistan.
| Email: |  |
Copyright © 2023 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

In this paper, the solubility of the components in the system (NH4)2HPO4-NH(C2H4OH)2-H2O was studied from the freezing point (-58.7°C) to 70.0°C. A polythermal solubility diagram has been constructed, on which the crystallization regions of ice, diammonium phosphate, diethanolamine and the compound (NH4)2HPO4·NH(C2H4OH)2 are demarcated. Visual-polythermal, quantitative chemical analysis, spectrophotometric and colourimetric methods were used to study the system. The thermal analysis of the studied new phases was carried out in the derivatography of the Paulik-Paulik-Erdey system. X-ray phase analysis was performed on a Dron-3.0 diffractometer. Based on the results of the study of binary systems and internal compartments, a polythermal diagram of the solubility of three systems was compiled, in which the crystallization areas of the compound containing ice, diammonium phosphate, two aqueous diammonium phosphates, diethanolamine and (NH4)2HPO4·NH(C2H4OH)2 were separated. In the system, it was found that diethanolammonium has a clear effect on diammonium phosphate and the compound, which increases with increasing temperature and concentration.
Keywords: Solubility, Crystallization, Diammonium phosphate, Diethanolamine, Absorption of basic nutrients
Cite this paper: Saidolim Mardanov, Shokhida Khamdamova, Solubility of Components in the (NH4)2HPO4-NH(C2H4OH)2-H2O System, International Journal of Materials and Chemistry, Vol. 13 No. 2, 2023, pp. 23-27. doi: 10.5923/j.ijmc.20231302.02.
1. Introduction
- In the development and intensification of cotton production, cotton defoliation plays a huge role as one of the important agrotechnical measures.Interest in studying the interaction of ethanolamines with fertilizer components, as well as chlorate-containing defoliants, is because when used together, the effectiveness of the synthesized drugs increases. Therefore, physicochemical studies of the interaction of ethanolamines with macro- and microcomponents of fertilizers are of significant theoretical and practical interest.Ethanolamines have a beneficial effect on the growth and development of plants, improve the absorption of basic nutrients, increase productivity and accelerate the ripening of various crops [1].When explaining the growth activity of ethanolamines, it should be taken into account that in the presence of carbon dioxide and oxygen, ethanolamines can form glycerol, glycol, oxalic, formic, naphthenic and acetic acids, which belong to the group of growth substances [2-3].Physiologically active substances based on ethanolamines and microelements provide high efficiency when used in agricultural production by spraying on cotton and wheat.To successfully defoliate cotton, preparations are needed that ensure a high degree of leaf fall and boll opening. One of the possible ways to solve this important problem is to obtain and use chlorate-containing defoliants for defoliation together with compounds containing the -CH2-CH2- ethylene group. These compounds include ethanolamines and their derivatives. By penetrating into plants, they increase the level of ethylene in the plant body. This contributes to the acceleration of defoliation and the full ripening and opening of cotton bolls [4-5].
2. Materials and Methods
- When studying the system, the visual-polythermal method was used [6].For quantitative chemical analysis of liquid and solid phases, elemental analysis for carbon, nitrogen, and hydrogen was used [7]; the P2O5 content was determined using a spectrophotometer using the colourimetric method [8]. The content of elemental carbon and hydrogen was carried out according to the method [9]. Solid phases were identified by chemical and various methods of physicochemical analysis. Thermal analysis of the new phases under study was carried out on a derivatograph of the Paulik-Paulik-Erdey system. X-ray phase analysis was carried out on a Dron-3.0 diffractometer. The values of interplanar distances were found from the reference book [10-11], according to the angle of reflection, and the intensity of diffraction lines was assessed on a 100-point scale.
3. Results and Discussion
- To characterize the behaviour of the components in a wide concentration and temperature range, the solubility of the (NH4)2HPO4-NH(C2H4OH)2-H2O system was studied using the visual-polythermal method [6] from the temperature of complete freezing (-58.7°C) to 70.0°C.We studied the solubility of the (NH4)2HPO4-NH(C2H4OH)2-H2O system [12-25] using eight internal cuts. Of these, sections I-III were studied from the side NH(C2H4OH)2-H2O to the top of (NH4)2HPO4, sections IV-VIII from the side (NH4)2HPO4-H2O to the top of NH(C2H4OH)2.Based on the results of studying binary systems and internal sections, a polythermal solubility diagram of the ternary system was constructed, on which the crystallization fields of ice, diammonium phosphate, dihydrous diammonium phosphate, diethanolamine and a compound of the composition (NH4)2HPO4·NH(C2H4OH)2 are delimited (Fig. 1).
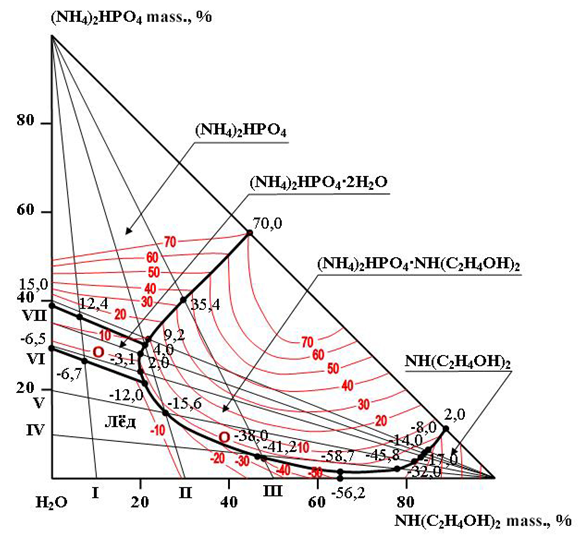 | Figure 1. A sample line graph using colours which contrast well both on screen and on a black-and-white hard copy |
|
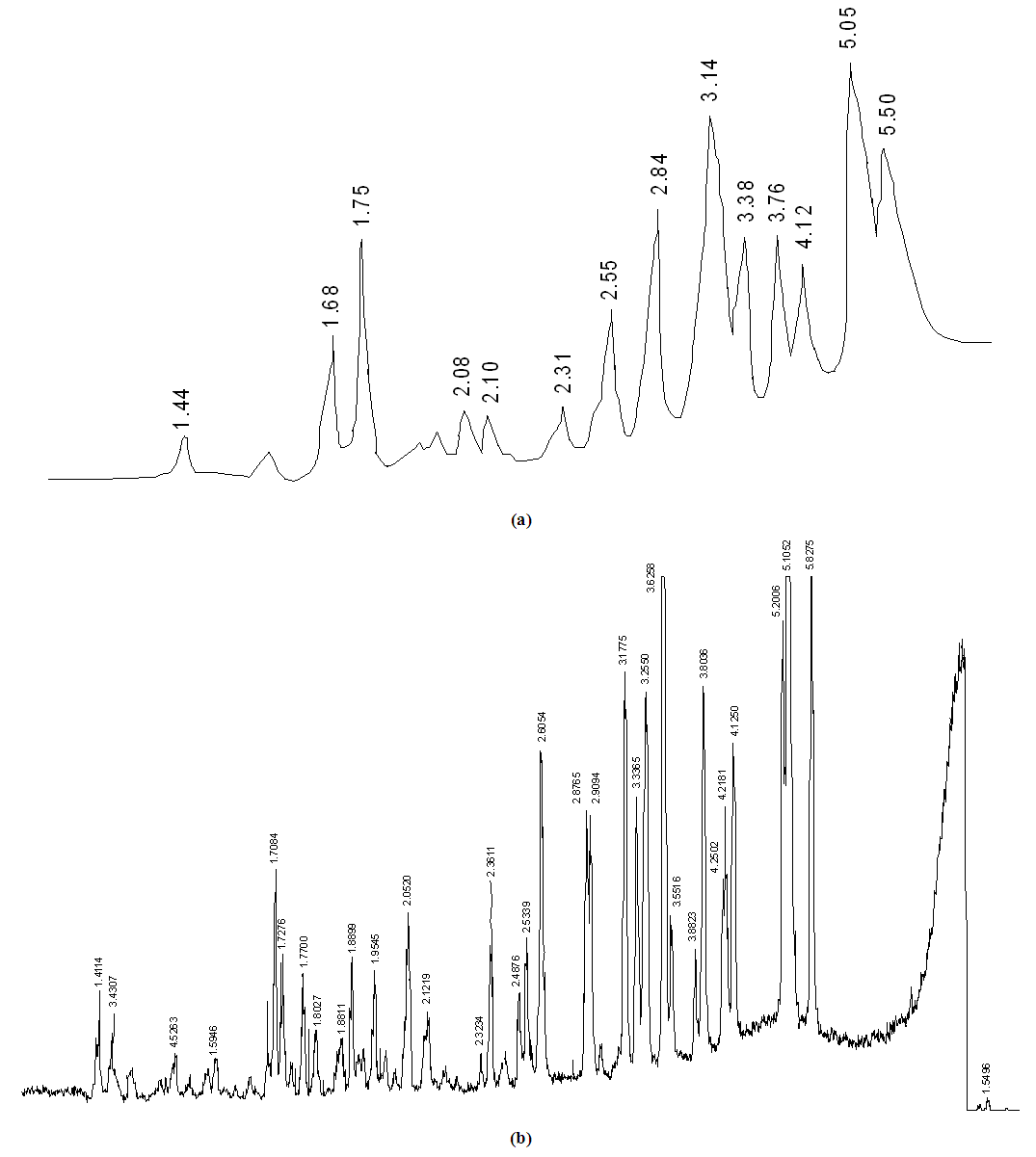 | Figure 2. X-ray diffraction patterns of (NH4)2HPO4 (a) and (NH4)2HPO4·NH(C2H4OH)2 2 (b) |
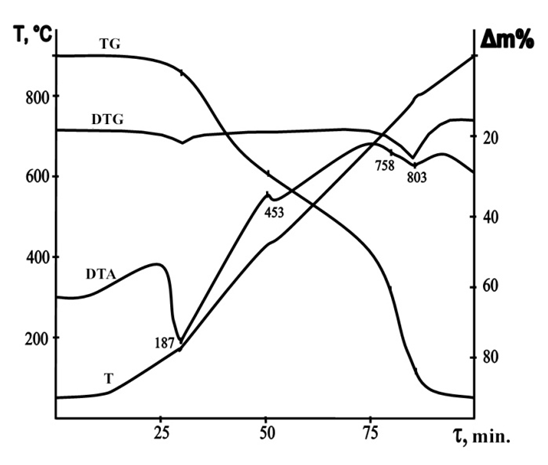 | Figure 3. Derivatogram (NH4)2HPO4·NH(C2H4OH)2 |
4. Conclusions
- From the solubility diagram, it is clear that on the liquidus surface a significant volume is occupied by the crystallization fields of diammonium phosphate and the new compound of the composition (NH4)2HPO4·NH(C2H4OH)2. This indicates their low solubility in the studied temperature and concentration ranges relative to other components of the system. The system revealed a clear salting-out effect of di-ethanol ammonium on diammonium phosphate and a compound that increases with increasing temperature and concentration.The results of the studied system indicate the possibility of obtaining a compound with physiologically active properties by jointly dissolving the initial components in those ratios where a minimal salting-out effect is observed.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML