-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
International Journal of Materials and Chemistry
p-ISSN: 2166-5346 e-ISSN: 2166-5354
2023; 13(1): 5-10
doi:10.5923/j.ijmc.20231301.02
Received: Apr. 13, 2023; Accepted: Apr. 28, 2023; Published: May 12, 2023

Kinetic Laws of the Reaction of Dimethyl Ether Synthesis from Synthesis-Gas
Jasur Shukurov1, Normurot Fayzullaev2
1Doctoral student of Samarkand State University, Samarkand, Uzbekistan
2Doctor of Technical Sciences, Professor, Department of Polymer Chemistry and Chemical Technology, Samarkand State University, Samarkand, Republic of Uzbekistan
Correspondence to: Jasur Shukurov, Doctoral student of Samarkand State University, Samarkand, Uzbekistan.
| Email: |  |
Copyright © 2023 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

In the article, more than 20 catalysts were tested for synthesis gas dimethyl ether. Among them, catalysts containing Al2O3*CuO*ZnO*ZrO2//HSZ and Cr2O3*Al2O3*ZnO//HSZ were proven to have high catalytic activity and productivity. In the presence of these catalysts, it was found that during the reaction, coke and tars are formed in minimal amount, and the catalyst works without changing its catalytic activity for a long time. Therefore, Al2O3*CuO*ZnO*ZrO2//HSZ and Cr2O3*Al2O3*ZnO//HSZ catalysts with the following composition were used for methanol synthesis. Two-flow devices were used to conduct catalytic experiments: 2-4 MPa in the pressure range and 0.2-0.6 MPA (low-pressure device) in the range. Catalyst activation was conducted in a nitrogen-hydrogen stream (≈2 Vol.% H2 at N2, consumption ≈2 l/h). Contact Time, s∙kgcat/l defined by the formula: τ=3600m/Vkir, conversion of methanol to dimethyl ether (DME) calculated by the formula: ХСН3ОН = 2СDME∙100(2СDME+ См). The conversion of methanol to DME rises as contact duration increases (volumetric velocity decreases). Pressure reduction reduces the rate of methanol production but does not affect methanol dehydration. As a result, the higher the methanol synthesis, the lower the pressure at the same contact time.
Keywords: Synthesis-gas, Methanol, Dimethyl ether, Volumetric rate, Temperature
Cite this paper: Jasur Shukurov, Normurot Fayzullaev, Kinetic Laws of the Reaction of Dimethyl Ether Synthesis from Synthesis-Gas, International Journal of Materials and Chemistry, Vol. 13 No. 1, 2023, pp. 5-10. doi: 10.5923/j.ijmc.20231301.02.
1. Introduction
- One of the most important processes in the chemical industry is the deep processing of natural gas, petroleum satellite gas, biogas, and others into valuable petrochemical products [1,2], and the conversion of natural and petroleum satellite gas into easily transportable products allows natural resources to be transferred to universal energy reserves [3-7]. In the years since scientists all around the world have been fascinated by the process of converting synthesis gas into dimethyl ether at one stage.The composition and properties of the ethylene series hydrocarbons obtained from dimethyl ether are determined by the physicochemical properties of the zeolite catalyst used in this process, texture characteristics, extraction methods and conditions, and the ratio of gas and hydrogen in the synthesis-gas composition. Controlling the acidic properties of high-silicon zeolites as well as the initial synthesis-gas composition allows for the production of a light sulfur-free synthetic oil from methanol and dimethyl ether [8-10]. The acidic properties of zeolites are known to be dependent on the nature of the exchange cations [11-13] and the technique of their introduction [14-16]. Nowadays, in commercial synthesis via methanol-in getting hydrocarbons such as ethylene, propylene, and butylenes from gas, ZSM-5 type catalysts [17] and Sapo-34 type molecular sieves are frequently utilized as catalysts [18]. It is known that the synthesis of hydrocarbons such as ethylene, propylene and butylenes from dimethyl ether is easier than the synthesis of hydrocarbons such as ethylene, propylene and butylenes from methanol [16-23], which is why dimethyl ether is a stronger methylating agent compared to methanol [8-13], which allows the reaction of the synthesis of unsaturated hydrocarbons from dimethyl ether to be conducted with a sufficiently large. Furthermore, dimethyl ether has less activity than methanol in the hydrogen displacement processes that result in the formation of Coke, resulting in a reaction to obtain unsaturated hydrocarbons from dimethyl ether with a significantly lower rate of catalyst deactivation than the reaction with methanol. Currently, it is critical to generate organic synthesis and petrochemical products based on methane and acetylene-based syntheses. It is mostly used to oxy-conduct methane, aromatize methane and propane-butane fractions, synthesize methanol and dimethyl ether from methane and synthesis gas, and produce lower molecular olefins from them. In these reactions, high-silicon zeolites are used as catalysts and trapping agents [25-41].
2. Experimental Part
- For methanol synthesis, the following catalysts were used: Al2O3*CuO*ZnO*ZrO2//HSZ and Cr2O3*Al2O3*ZnO//HSZ. A chromatographic approach was used to evaluate the amount of chemicals at the reactor's outlet. Catalytic studies were carried out using two flow devices: 2-4 MPa in the pressure range and 0.2-0.6 MPA (low-pressure device) in the range. The reactor's inner diameter is 11 mm, while the thermocouple channel's outer diameter is 4 mm. The catalyst torque was 0.5 g mass, with a fraction of 0.25-0.315 mm. The temperature of the catalytic floor is measured using two stainless steel thermocouples (wall thickness 0.25 mm) (the diameter of the thermocouple at the entrance and exit from the floor is 1 mm). A Porapak T colon is used to determine DME, methanol, and water. Catalyst activation was carried out in a nitrogen-hydrogen current (2 Vol.% H2 at N2, consumption 2 l/h). When conducting catalytic tests, synthesis is performed-gas composition and volume %: СО–21,8; СО2–5,2; N2–5,3: H2–67,7.Contact time, s•kgkat/l, determined by the formula:
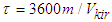 Where m is the tensile mass, kg; - load on raw materials brought to normal conditions, l/h.The conversion of methanol to DME was calculated using the formula:
Where m is the tensile mass, kg; - load on raw materials brought to normal conditions, l/h.The conversion of methanol to DME was calculated using the formula: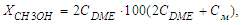 Here СDME, См- volumetric concentrations of DME and methanol at the exit of the reactor, respectively.X-ray phase analysis (XPA) of the samples was performed on a Shimadzu XRD-7000 diffractometer in CuKα radiation (λ = 1.5418 Å). The morphology of the samples was studied using a JEOL JSM 6390 LA scanning electron microscope (SEM) (Japan).
Here СDME, См- volumetric concentrations of DME and methanol at the exit of the reactor, respectively.X-ray phase analysis (XPA) of the samples was performed on a Shimadzu XRD-7000 diffractometer in CuKα radiation (λ = 1.5418 Å). The morphology of the samples was studied using a JEOL JSM 6390 LA scanning electron microscope (SEM) (Japan).3. Results and Discussion
- Compositions including methanol (methanol synthesis catalyst) and dehydrating (methanol dehydration catalyst) components are commonly utilized in the direct synthesis of DME. At 2-4 MPa and 260-320°C, the kinetics of direct synthesis of DME from synthesis gas were investigated in bifunctional catalysts including Al2O3*CuO*ZnO*ZrO2 // HSZ and Cr2O3*Al2O3*ZnO//HSZ. The volume decreases during the synthesis of dimethyl ether from gas and hydrogen, thus the process was carried out at high temperatures; the influence of temperature on the yield of dimethyl ether is illustrated in Figure 1. It can be seen that the conversion of carbon dioxide increases with increasing pressure, with the production of dimethyl ether having the greatest value at a pressure of 1 MPa (Fig. 1).
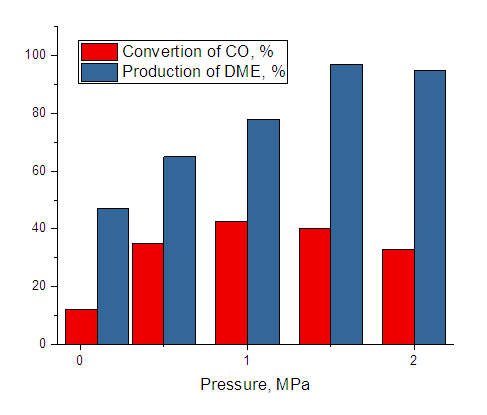 | Figure 1. Effect of pressure on CO gas conversion and dimethyl ether yield (T=300°C, hydrogen: Carbon monoxide=2, volumetric rate 1000 hour-1.) |
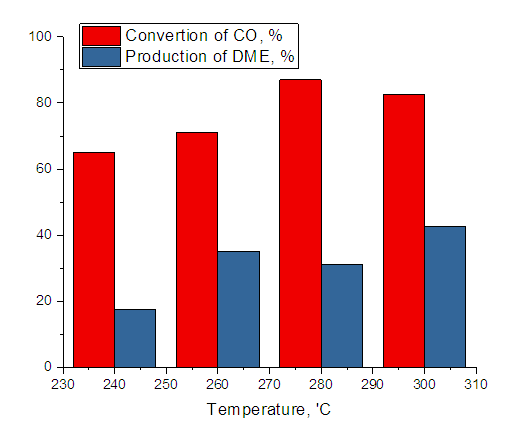 | Figure 2. Effect of temperature on carbon monoxide conversion and dimethyl ether yield (T=300°C, hydrogen: Carbon monoxide=2, volumetric rate1000 hour-1.) |
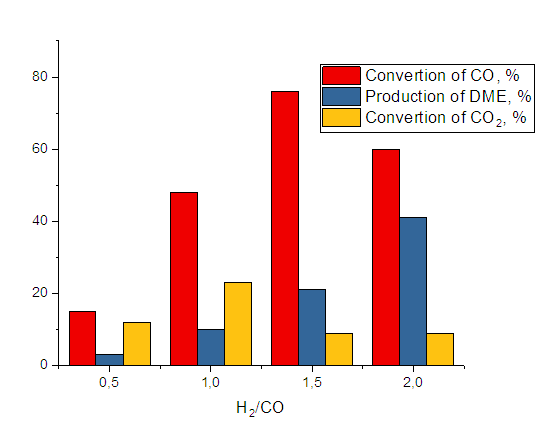
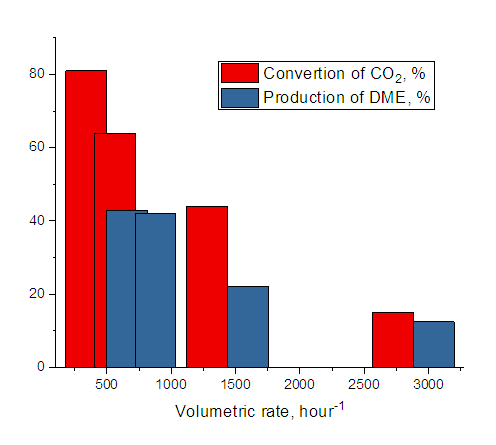 | Figure 3. Effect of volumetric rate on the conversion of Carbon monoxide gas and dimethyl ether yield P=1 MPa, T=300°C, hydrogen: Carbon monoxide, volumetric rate1000 hour-1 |
 | Figure 5. Al2O3*CuO*ZnO*ZrO2//HSZ and Cr2O3*Al2O3*ZnO//HSZ catalysts (1:1) |
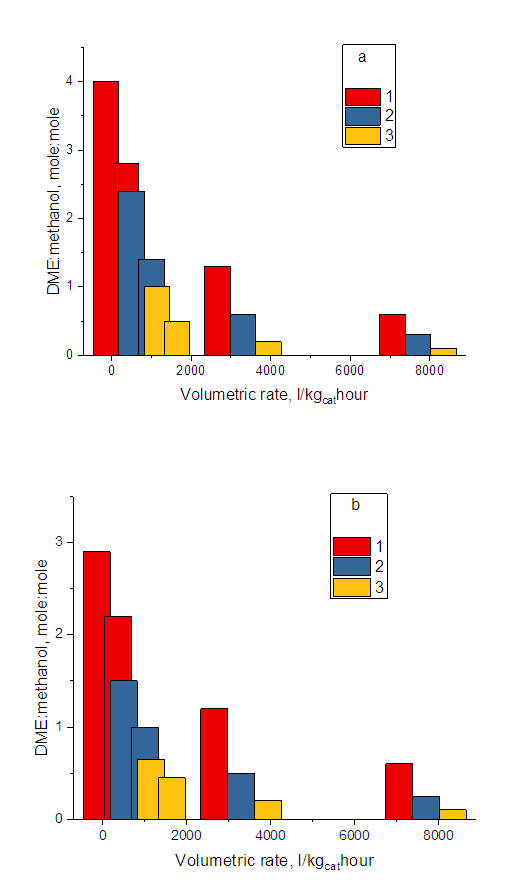 | Figure 6. DME: methanol mole ratio dependence on the volumetric rate. MPa pressure: a - 2; b - 4. Temperatures in degrees Celsius: 1 -320; 2 -300; 3 -280 |
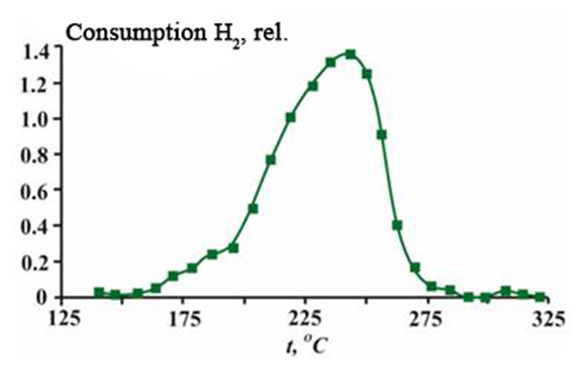 | Figure 7. Temperature programmed reduction spectrum of CuO*ZnO*Al2O3*ZrO2/HSZ catalyst |
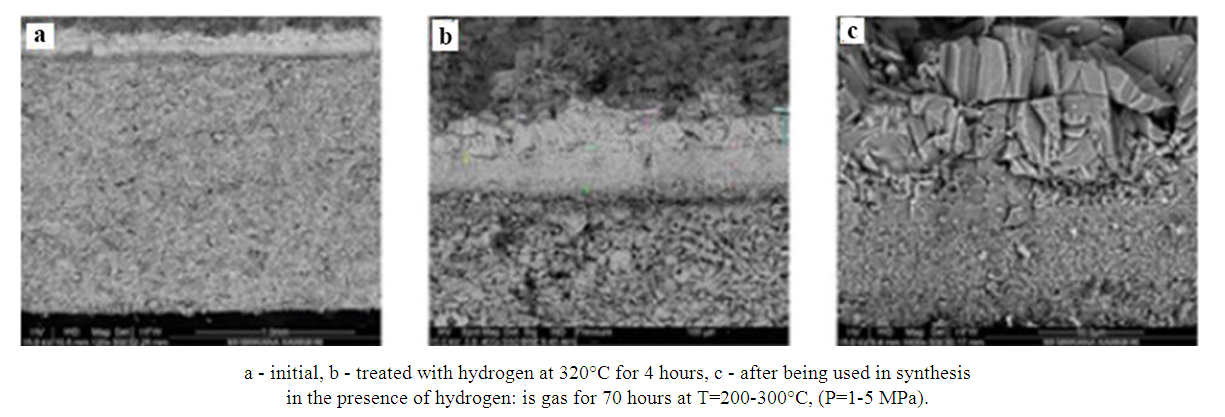 | Figure 8. Micrographs of Al2O3*CuO*ZnO*ZrO2//HSZ sample |
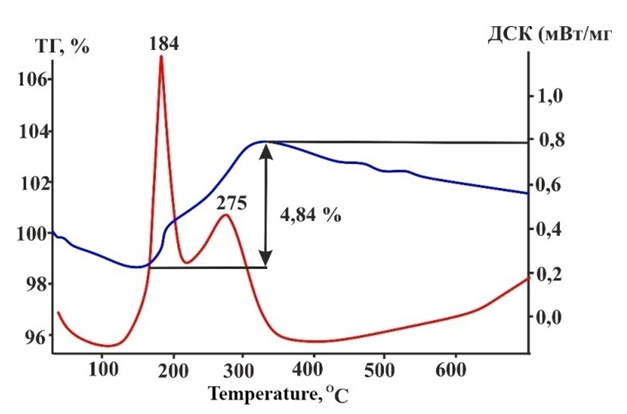 | Figure 9. Differential thermal analysis of treated catalyst sample after 70 hours of use |
4. Conclusions
- The conversion of methanol to DME increases as the contact time grows (volumetric rate lowers), approaching the thermodynamic equilibrium value. A decrease in pressure reduces the rate of methanol production but has no effect on methanol dehydration. As a result, the higher the methanol synthesis, the lower the pressure at the same contact time. The presence of methanol in the reaction products is caused by the thermodynamics of the dehydration reaction. The DME: methanol ratio can be changed by changing the direct synthesis conditions.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML