-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
International Journal of Materials and Chemistry
p-ISSN: 2166-5346 e-ISSN: 2166-5354
2023; 13(1): 1-4
doi:10.5923/j.ijmc.20231301.01
Received: Jan. 26, 2023; Accepted: Feb. 20, 2023; Published: Feb. 28, 2023

Catalytic Activity of the Ce0.72Zr0.18Pr0.1O2 and Pt/Ce0.72Zr0.18Pr0.1O2 Systems in the Oxidation of Carbon Monoxide
Yuldashev Khayot Khurmatovich1, Kodirova Dilshodxon Tulanovna1, Mansurov Yulbarskhon Nabiyevich2
1Department of Chemistry and Chemical Technology, Fergana Polytechnic Institute, Fergana, Uzbekistan
2Tashkent State Transport University, Tashkent, Uzbekistan
Correspondence to: Yuldashev Khayot Khurmatovich, Department of Chemistry and Chemical Technology, Fergana Polytechnic Institute, Fergana, Uzbekistan.
| Email: |  |
Copyright © 2023 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

In this work, samples of the Ce0.72Zr0.18Pr0.1O2 support and systems deposited on their surface with 0.5, 1.0 and 2.0% (wt.) of platinum were obtained. Studies were carried out on the catalytic properties of the samples during the oxidation of carbon monoxide by gas chromatography. The research on the catalytic activity of the obtained samples in the oxidation of carbon monoxide was carried out in a flow reactor under appropriate conditions. The study of the catalysts showed high activity in the oxidation of CO in the presence of platinum.
Keywords: Platinum, Platinum acetylacetonates, Cerium oxide, Zirconium oxide, Praseodymium oxide, Catalysis, Catalytic oxidation
Cite this paper: Yuldashev Khayot Khurmatovich, Kodirova Dilshodxon Tulanovna, Mansurov Yulbarskhon Nabiyevich, Catalytic Activity of the Ce0.72Zr0.18Pr0.1O2 and Pt/Ce0.72Zr0.18Pr0.1O2 Systems in the Oxidation of Carbon Monoxide, International Journal of Materials and Chemistry, Vol. 13 No. 1, 2023, pp. 1-4. doi: 10.5923/j.ijmc.20231301.01.
Article Outline
1. Introduction
- Today, the issue of air pollution with harmful substances during the operation of vehicles with internal combustion engines is relevant. Every year the number of vehicles is growing, while the content of emissions in the atmospheric air is rising. To neutralize harmful gaseous emissions of internal combustion engines from CO, NOx, and CHx in cars, three-ways catalysts – TWC are used [1-5]. Cerium dioxide, as well as platinum, has found wide application in the processes of purification from harmful emissions from gas engines of internal transport [7–9] and connection with the operations of ecological catalysis [11]. Due to its well-known unique redox properties and high mobility of oxygen in the lattice, cerium oxide is considered a promising component for such catalytic materials [3,10]. Particles of Ce-Zr dioxide systems also have more resistance to sintering, which prevents a decrease in the specific surface area of the catalyst under the action of heating and contributes to the preservation of its activity under high-temperature conditions. Ce-Zr dioxide systems modification with praseodymium ions leads to an increase in defect formation to grow in the oxygen mobility in the crystal lattice [6,9,12-18], which leads to growth in its catalytic activity. According to [15], adding even a small amount of Pr can increase the oxygen mobility of CeO2.This work aimed to study and compare the catalytic properties of Ce0.72Zr0.18Pr0.1O2 and Pt/Ce0.72Zr0.18Pr0.1O2 samples in the process of CO oxidation to reduce vehicle emissions into the environment.
2. Materials and Methods
- Ce0.72Zr0.18Pr0.1O2 solid solutions were synthesized according to the procedure described in [20]. Ce0,72Zr0,18Pr0,1O2 solid solutions were synthesized by coprecipitation of sparingly soluble compounds of cerium, zirconium, and praseodymium, followed by filtration, washing out of nitrate ions, drying, and calcination. The initial solution was prepared by mixing appropriate amounts of 0.1 M solutions of cerium (III), zirconyl, and praseodymium (III) nitrates in the molar ratio of Сe:Zr: Pr = 0,72:0,18:0,1, respectively. The solution was heated to 40°C, after which, with constant stirring, a 5% ammonium hydroxide solution was slowly added until pH=10-11 was reached. Next, the precipitates were subjected to ageing under a layer of mother liquor for 1 h at room temperature. The precipitate was separated by centrifugation, dried at 100°C for 20 hours, and then calcined in a muffle furnace at 500°C for 2 hours.The supported Pt/Ce0.72Zr0.18Pr0.1O2 catalysts were obtained by impregnation from platinum (II) acetylacetonate. The dried weighing of oxide carrier weighing 5 g was introduced into a solution containing the required amount of platinum (II) acetylacetonate in 20 ml of methylene chloride. Then the mixture was dried with constant stirring in a stream of warm air until a mass was obtained in the form of a free-flowing powder. The resulting sample was calcined in a muffle furnace at 300°C with atmospheric air and then reduced in a stream of hydrogen H2 at 400°C for 2 hours.The catalytic activity of the obtained samples in the CO oxidation reaction was studied by the flow method. The composition of the model gas mixture was (vol.%): СО – 3,35; О2 – 7,98; N2 – 88,67. The concentrations of CO and O2 were measured on a Chrom-5 gas chromatograph (13X molecular sieves with a length of 1.5 m and an inner diameter of 4 mm on the sorbent). Regeneration of the column was carried out for 20 min at a temperature of 150°C. The temperature of the columns during measurements was 60°C. The tests were carried out at a gas mixture space velocity of 1800 h-1 in the temperature range from minus 13°C to 300°C.
3. Results and Discussion
- The results of the catalytic activity by the oxidation of CO are illustrated in Fig. 1. From the analysis of Fig. 1, it follows from Table 1 that sample containing platinum, which provides complete oxidation of CO in the low-temperature region, showed high activity in the model reaction of CO oxidation. The 50% conversion temperature is: 33°C for the system with 2 wt % Pt, 197°C for the pure support sample, and the 90% conversion temperature is 52°C and 250°C for Pt/Ce0.72Zr0.18Pr0.1O2 and Ce0.72Zr0.18Pr0.1O2, respectively.
|
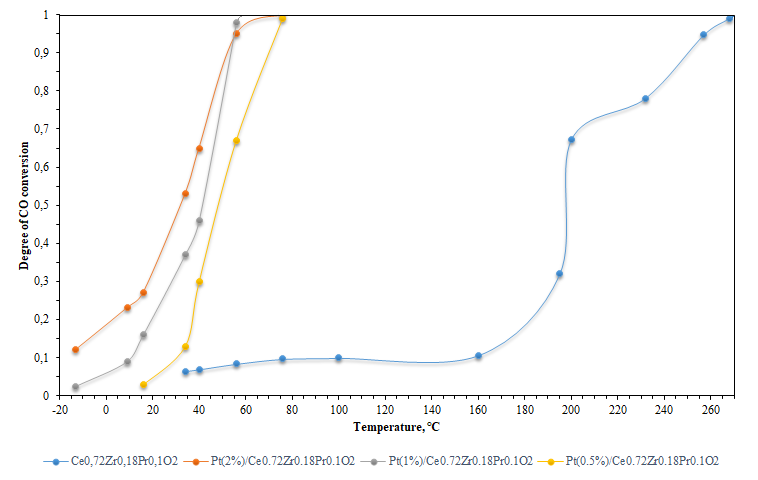 | Figure 1. The catalytic activity of the obtained samples in the reaction of CO oxidation |
4. Conclusions
- In the study of the catalytic activity of the obtained samples in the CO oxidation reaction, both models showed positive results in the oxidation of CO, but the application of 2% (wt.) Pt greatly improves the catalytic activity of the support. Samples of the PtOx-CeO2 solid solution with a platinum content of 2% (wt.) have catalytic activity in the oxidation of CO at temperatures above 180°C, and the sample synthesized in this work of 2% (wt.) Pt/Ce0.72Zr0.18Pr0.1O2 has such activity at a temperature of 52°C. Models of the solid solution of oxides Ce0.8Zr0.1Pr0.1O2 [13] and Ce0.8Zr0.1Pr0.1O2 [19] have a specific activity of 90% CO conversion at temperatures of 295°C and 387°C, respectively, respectively, while the synthesized sample of Ce0.72Zr0.18Pr0.1O2 has such activity at a temperature of 250°C. The Pr0,1Zr0,18Ce0,72O2 model obtained in [11] has a similar performance. The temperature of 50% CO conversion differs by 9 degrees and that of 90% conversion by 4 degrees.
ACKNOWLEDGEMENTS
- The authors acknowledge the immense help received from the scholars whose articles are cited and included in references to this manuscript. The authors are also grateful to the authors/ editors/publishers of all those articles, journals and books from where the literature for this article has been reviewed and discussed.The authors report no conflicts of interest. The Source of funding is nil.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML