-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
International Journal of Materials and Chemistry
p-ISSN: 2166-5346 e-ISSN: 2166-5354
2022; 12(3): 49-53
doi:10.5923/j.ijmc.20221203.03
Received: Nov. 28, 2022; Accepted: Dec. 19, 2022; Published: Dec. 23, 2022

Obtaining Food Additives Based on Local Plant Waste and Determination of the Quantity of Polysaccharides in Their Composition by the Physico-Chemical Method
Ibragim R. Askarov1, Sultanoy Kh. Mikhmanova2, Nodira Kh. Abdurakhimova3, Isakov Khayatulla1
1Doctor of Chemical Sciences, Andijan State University, Andijan, Republic of Uzbekistan
2Freelance Researcher, Andijan State University
3Lecturer, Andijan State University, Andijan, Republic of Uzbekistan
Copyright © 2022 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

The contents of seeds and pods of melons were extracted with water and the amount of polysaccharides was studied. Based on the results obtained, it was determined that Water-soluble polysaccharide- (WSP), 7.5%, Pectin substances (PS)- 2.5% and Hemicelluloses (HMC) - 2.1%. When studying the composition of the seeds, it was found that WSP is 7%, PS 2% and HMC 0.5%. In the study of the obtained samples by IR spectroscopy, characteristic absorption peaks of Glc, Gal, Ara, Man, Xyl, and Rha are formed.
Keywords: Peels and seeds, Alcohol-soluble sugars, Water-soluble polysaccharides, Pectin substances, Hemicelluloses, IR spectroscopy
Cite this paper: Ibragim R. Askarov, Sultanoy Kh. Mikhmanova, Nodira Kh. Abdurakhimova, Isakov Khayatulla, Obtaining Food Additives Based on Local Plant Waste and Determination of the Quantity of Polysaccharides in Their Composition by the Physico-Chemical Method, International Journal of Materials and Chemistry, Vol. 12 No. 3, 2022, pp. 49-53. doi: 10.5923/j.ijmc.20221203.03.
Article Outline
1. Introduction
- The extraction of food additives from melon by-products is of great importance for food safety. Currently, more than 160 cultivars of melon are grown and exported to foreign countries in Uzbekistan [1].Melon varieties grown in Uzbekistan consist of various chemical compounds. These include fats, vitamins, polysaccharides and others. For example, melon peel is very sweet due to its high fructose content. These properties determine the value of melon in terms of its medicinal properties and use in folk medicine [2].The rich chemical composition of the Bacchanalian cultures is evidenced by the data of studies by various scientists [1-5].The main indicator of melon quality is its chemical composition. Water is the main component of melon and, depending on the variety of culture, its content is determined in the range of 84-88.5%. The composition of substances contained in melon includes proteins, carbohydrates (sugars, starch, fiber), organic acids, vitamins, minerals. The chemical composition of fruits is largely determined by the soil and climatic conditions of cultivation, the level of agricultural technology, the correctness and timing of the use of irrigation mode, the timeliness of collection, the organization of the storage regime, the preparation of products for storage. Based on the above, it should be noted that the most important stage in the development of technology for the production of long-term storage products of increased food and biological value is the determination of the chemical composition of melon.
2. The Main Findings and Results
- The aim of the research is to isolate polysaccharides (WSP and pectin substances, hemicellulose and sugar) contained in the peel and seeds of melon, and to determine their composition by physico-chemical methods.Objects and methods of research. A carbohydrate complex (crusts and seeds) growing in Uzbekistan has been studied. As a result of the study, the presence of alcohol-soluble sugars, water-soluble polysaccharides, pectin substances and hemicelluloses was established. IR spectra of isolated polysaccharides were also studied.10 g of crushed air-dry raw materials were extracted with boiling chloroform in a ratio of 1:8 in a round-bottomed flask with a reverse refrigerator to remove coloring and low-molecular compounds [6]. Extraction was carried out three times, after which the raw materials were separated by filtration and dried. Isolation and study of alcohol-soluble sugars. The dried raw materials were extracted with boiling 82% ethanol (1:10, 1:6) in a round-bottomed flask with a reverse refrigerator. Extraction was performed twice. Alcohol extracts were combined, evaporated on a rotary evaporator to a small volume and chromatographed on Filtrak-FN-13 paper for 18 hours by a descending method in a butanol-pyridine-water solvent system (6:4:4) in comparison with known monosaccharide samples. For the manifestation of hexosaccharochromatograms, acidic aniline phthalate was shown and heated in a drying cabinet at 105°C for 2-3 minutes. For the manifestation of ketosaccharides, a 5% alcohol solution of acidified urea was used, followed by heating them in a drying cabinet at 105°C.Isolation and study of water-soluble polysaccharides (WSP). The remainder of the raw material after the isolation of alcohol-soluble sugars was extracted twice, with a hydromodule 1:15, 1:10 (600, 500 ml of water) in a water bath at 70-75°C, stirring constantly. Each extract was separated by filtration through calico under vacuum. The extracts were combined, evaporated on a rotary evaporator to 40 ml and precipitated with alcohol (1:3). The precipitate was separated by centrifugation (5000 rpm, 10 min), dried and washed with alcohol. Hydrolysis of WSP. 100 mg of isolated WSP were hydrolyzed with 3 ml of sulfuric acid solution (1 mol/l) in a sealed ampoule in a boiling water bath for 8 hours at 100°C. After the specified time, the ampoule was opened, the hydrolysate was placed in a glass with a capacity of 50 ml and neutralized with barium carbonate. The precipitate formed in this case was filtered out, the filtrate was deionized with KU–2(H*) cationite, evaporated to a small volume (0.5 ml) and chromatographed on Filtrak-FN-12,13 paper by a descending method in a solvent system butanol-pyridine-water (6:4:3) with known monosaccharides ("witnesses") Chromatograms were dried, developed with acidic aniline phthalate, followed by heating in a drying cabinet at 100°C 1-2 min. Isolation and study of pectin substances (PS). The remainder of the raw material after extraction of the WSP was treated twice with 300 ml of a mixture of 0.5% solutions of oxalic acid and ammonium oxalate (1:1) with a godromodule (1:15, 1:10) at 70-75°C for 1 hour, with stirring. The obtained extracts were separated by filtration through calico, combined and dialized against running water for 18 hours.. Then they were evaporated on a rotary evaporator to 50 ml and precipitated with alcohol (200 ml). The precipitate was separated by centrifugation (5000 rpm, 10 min), the precipitate was washed with alcohol and dried. Hydrolysis of PS. 100 mg of PS was hydrolyzed with 3 ml of sulfuric acid solution (2 mol / l) in an ampoule in a boiling bath for 24 hours. The method of processing the hydrolysate and its analysis are described above.Isolation and study of hemicelluloses. Hemicelluloses (HMC) were isolated from the remaining raw materials (after extraction of PS) by double extraction with 5% sodium hydroxide solution (1:10, 1:5) at room temperature, stirring constantly for 2 hours. The extracts were separated by filtration, combined, neutralized with 50% acetic acid solution, dialized against running water for 20 hours., then evaporated and precipitated with alcohol. Hydrolysis of HMC. 100 mg HMC was hydrolyzed with 3 ml of sulfuric acid solution (2 mol/l) in an ampoule in a boiling water bath for 48 hours. The hydrolysate was processed and analyzed according to the procedure described above. Analysis of isolated polysaccharides by IR spectroscopy. IR spectra of polysaccharides were taken on a Perkin-Elmer, FT-IR/NIRSpectrometr Fourier infrared spectrometer. Spectrium 3. Universal ATR Sampling Accessory of the absorption region (range) 530-3600 cm-1 [8].Hemicelluloses analysis of samples was carried out on a ShimadzuGC-2010 chromatograph with flame ionization detector, ShimadzuRxi-624SilMS quartz capillary column (30mx0.25mmx1.40mkm), mobile phase velocity (N2) 1.5 ml/min, injector temperature 260°C, detector temperature 280°C and column temperature 230°C. Samples were taken in the form of aldononitrile acetates [9].
3. Results and Discussion
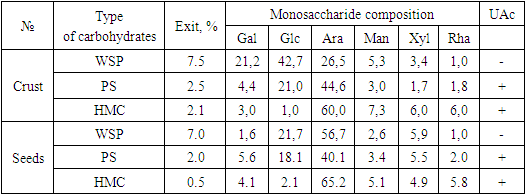
- As a result of the study, it was found that alcohol–soluble sugars and seeds are represented by hexose - glucose (brown spot with Rf = 0.36), ketosaccharides fructose and sucrose (blue spots with Rf= 0.60 and Rf= 0.46, respectively).The yield of water-soluble polysaccharides (WSP) was 0.75 g (7.5%) and 0.7 g (7.0%) crusts and seeds. WSP are amorphous powders of light beige color, well soluble in water. The monosaccharide compositions of WSP did not differ dramatically qualitatively, but the difference was in the quantitative ratio. The main monosaccharides of WSP -x are Ara, Glu, and Gal, and in WSP -g are Ara, Glu; other monosaccharides are represented in smaller quantities. The ratio of monosaccharides allows us to assume that the basis of the PS from the crust is made up of heterogeneous polysaccharides with a dominant content of glucans, and the presence of both glucans and glucoarabinans is possible in the PS of seeds [7].The yield of pectin substances (PS) was melon crusts 0.25 g (2.5%) and 0.2 g (2.0%) of seeds. PS is an amorphous white powder, well soluble in water. The PS solution gives with iodine a barely noticeable rapidly disappearing blue staining.It is shown that the monosaccharide composition of pectin substances is represented by galacturonic acid (Rf=0.14), galactose (Rf=0.37), arabinose (Rf=0.48), in small quantities (chromatographic zones were fuzzy and had a weak color) xylose (Rf=0.56) and rhamnose (Rf=0.67).The yield of HMC was melon peels 0.1 g (1.0%) and 0.05 g (0.5%) of seeds. Hemicelluloses are an amorphous beige powder, insoluble in water, well soluble in dilute alkalis.Chromatographic analysis of hemicelluloses revealed the presence of glucuronic acid (Rf=0.14), galactose (Rf=0.37), arabinose (Rf=0.48), xylose (Rf=0.56), in smaller amounts of glucose (Rf=0.36) and rhamnose (Rf=0.67) [8].Table 1 summarizes the data on the quantitative content and monosaccharide composition of the isolated polysaccharides.
|
 | Figure 1. WSP crusts (A) and Seeds (B) |
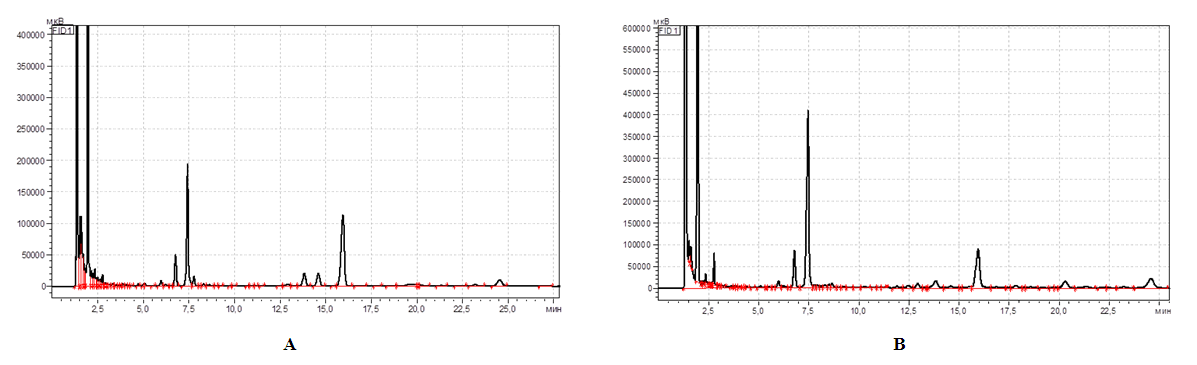 | Figure 2. IR spectra of Pectin substances in crusts (A) and Seeds (B) |
 | Figure 3. IR spectra of Pectin substances in crusts (A) and Seeds (B) |
4. Conclusions
- The contents of melon seeds and pods were extracted with water and the amount of polysaccharides was studied. Based on the results obtained, it was determined that the WSP is 7.5%, the PS is 2.5% and the HMC is 2.1%. When studying the composition of seeds, it was found that the WSP is 7%, PS is 2% and HMC is 0.5%. When studying the obtained samples by IR spectroscopy, characteristic absorption peaks of Glc, Gal, Ara, Man, Xyl and Rha are formed.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML