-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
International Journal of Materials and Chemistry
p-ISSN: 2166-5346 e-ISSN: 2166-5354
2022; 12(3): 44-48
doi:10.5923/j.ijmc.20221203.02
Received: Sep. 28, 2022; Accepted: Oct. 19, 2022; Published: Oct. 31, 2022

Physico-Chemical Analysis of the Complex of Furfurolidendiurea with Copper Acetate
Askarov R. Ibrokhim1, Isakov Khayatulla2, Mukhammedov B. Saidmurod3
1Doctor of Chemical Sciences, Andijan State University, Andijan, Uzbekistan
2Doctor of Technical Sciences, Andijan State University, Andijan, Uzbekistan
3Basic Doctoral Student, Fergana Polytechnic Institute, Fergana, Uzbekistan
Correspondence to: Mukhammedov B. Saidmurod, Basic Doctoral Student, Fergana Polytechnic Institute, Fergana, Uzbekistan.
| Email: |  |
Copyright © 2022 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

The article presents the results of physical and chemical tests to confirm the composition and individuality of the complex compound of biogenic metallic copper acetate salt of furfurolidendiurea with high fungicidal and stimulating properties, obtained on the basis of local raw materials. IR-spectroscopic, Mass-spectroscopic, scanning electron microscopic and thermal analyzes were used.
Keywords: Furfurolidendiurea, Fungicide, Biologically active, Furfurol
Cite this paper: Askarov R. Ibrokhim, Isakov Khayatulla, Mukhammedov B. Saidmurod, Physico-Chemical Analysis of the Complex of Furfurolidendiurea with Copper Acetate, International Journal of Materials and Chemistry, Vol. 12 No. 3, 2022, pp. 44-48. doi: 10.5923/j.ijmc.20221203.02.
1. Introduction
- Today, biologically active substances are considered important in the cultivation of high and quality crops from the fields on a global scale. Biologically active substances have a complex effect on the plant, at the same time fight against disease-causing bacteria and viruses, and have a positive effect on their growth and development. Therefore, it is an important task to provide agriculture with economically effective and less toxic biologically active substances much attention is being paid to its release. [1, 86-87p] Accordingly, in Uzbekistan, in recent years, great attention has been paid to the production of substances that protect plants and have a complex effect on their growth and development. Because today most of the preparations used against wilt and root rot, which are common in cotton plantations, and black fungus in grain crops, are used despite the fact that most of them are very toxic.It is very important to replace them with biologically active substances obtained on the basis of low-toxicity, ecologically harmless, inexpensive and, most importantly, local raw materials. [2, 4-5p] This research work is also being conducted on this topic.In order to obtain low-toxic and biologically active substances with a complex effect, furfurol obtained from agricultural waste with high fungicidal properties, urea, a typical representative of nitrogen fertilizers, and copper acetate salt, a biogenic metal, are used. As a result of the cross-linking of furfural and urea, the furfurolidendiurea compound is formed. [3, 32-34 p] To further increase the biological activity and effect of furfurolidendiurea on plants, it is possible to obtain a complex of copper with acetate salt. Copper and its salts have fungicidal, bactericidal and stimulating properties. [4, 44-47 p]
2. Experimental Part
- Obtaining the complex of furfurolidendiurea with copper acetate. We used copper acetate salt, one of the biogenic metals, to further increase the biological activity of furfurolidendiurea, obtained from the combination of furfurol and urea in the ratio of 1:2, obtained on the basis of agricultural waste, and to have a complex effect on plants. In this case, the same 0.5 molar solutions of furfurolidendiurea and copper acetate are prepared in 2 flasks of the same size. Both solutions were stirred at room temperature (20-25°C) for 2 hours using a reciprocating magnetic stirrer. After thorough mixing, the solution is poured into crystallizers. After the sample stands at room temperature for 1-2 days, the compound precipitates under the crystallizers. The precipitate is filtered, recrystallized and subjected to column chromatography to obtain the complex compound. [5, 77-78 p]Analysis of the obtained complex based on physico-chemical experiments. IR-spectroscopic analysis of the complex of furfurolidendiurea with copper acetate. In the implementation of this experiment, "IRTracer-100" (SHIMADZU CORP., Japan, 2017) total internal reflection (ATR) coupling MIRAcle-10 diamond/ZnSe prism (spectral fluctuations on the wave number scale - 4000÷400 cm-1; dimensions - An Infrared Fourier spectrometer with a sensitivity of 4 cm-1, a signal-to-noise ratio of 60,000:1, and a scanning speed of 20 spectra per second) was used. As a result of the analysis of IR spectra, it is possible to obtain additional information on the structural state by determining the characteristic absorption points of the functional groups that make up the substance. [6, 45-48 p]Examination of the complex in a scanning electron microscope. SEM - EVO MA 10 (Zeiss, Germany) scanning electron microscope was used to study the morphological appearance of the sample crystal and determine the mass percentages of the elements that make up the composition. First, a sample was prepared when performing experiments on a scanning electron microscope. In the preparation of the sample, it is placed in round containers made of a metal alloy with a diameter of 1 cm and a thickness of 1-3 mm, it is made into granules under pressure, and it is covered with a double-sided adhesive carbon film. During the measurement, 20.00 kV accelerating voltage (EHT - Extra High Tension) was used, the working distance (WD-working distance) was 8.5 mm. The measurement was performed in the secondary electron detection mode (SE 1-secondary electron detector). The appearance of the sample was imaged by SmartSEM at a scale of 100 microns.Also, the composition of the elements was determined by the X-ray spectroscopy (EDS) method using a SEM - EVO MA 10 (Zeiss, Germany) scanning electron microscope, in which they were used with an energy dispersive elemental analyzer - Aztec Energy Advanced X-act SDD. [7, 205- 208 p]Mass spectrometric analysis. Mass spectrometry is one of the most accurate methods for identifying substances. Mass spectrometric analysis of the compound of furfurolidendiurea with copper acetate was carried out by ionization with molecular nitrogen ions on a Perkin Elmer AxION 2 TF brand spectrometer. Some of the mass spectra recorded by the method of ionization of solid substances with ions in the plasma state may not correspond to the fragments contained in the substance. [8, 704 p] First, the sample was introduced into the ionization source, where the molecules were ionized. Then the newly formed positive ions accelerated under the influence of the electric field. Neutral molecules were removed with a vacuum pump. The stream of accelerated ions entered the mass analyzer, where the ions were separated by mass. The separated ion beam enters the detector, where the ion current is converted into an electrical signal, which is amplified and recorded.Thermal analysis. Thermoanalytical analysis of the complex was also performed. A Netzsch Simultaneous Analyzer STA 409 PG (Germany) was used for this analysis. A K-type thermocouple (Low RG Silver) was conducted with aluminum and a crucible. The measurements were carried out in an inert nitrogen atmosphere with a nitrogen flow rate of 50 ml/min and using air. [9, 48-52 p]
3. Results and Their Discussion
- In the IR-spectroscopic analysis of the compound of furfurolidendiurea with copper acetate, the desired results were obtained from the point of view of the structure of the molecule based on the determination of the relevant absorption frequencies of the functional groups that made up the sample. Figure 1 below shows the IR spectroscopic image of the obtained compound.
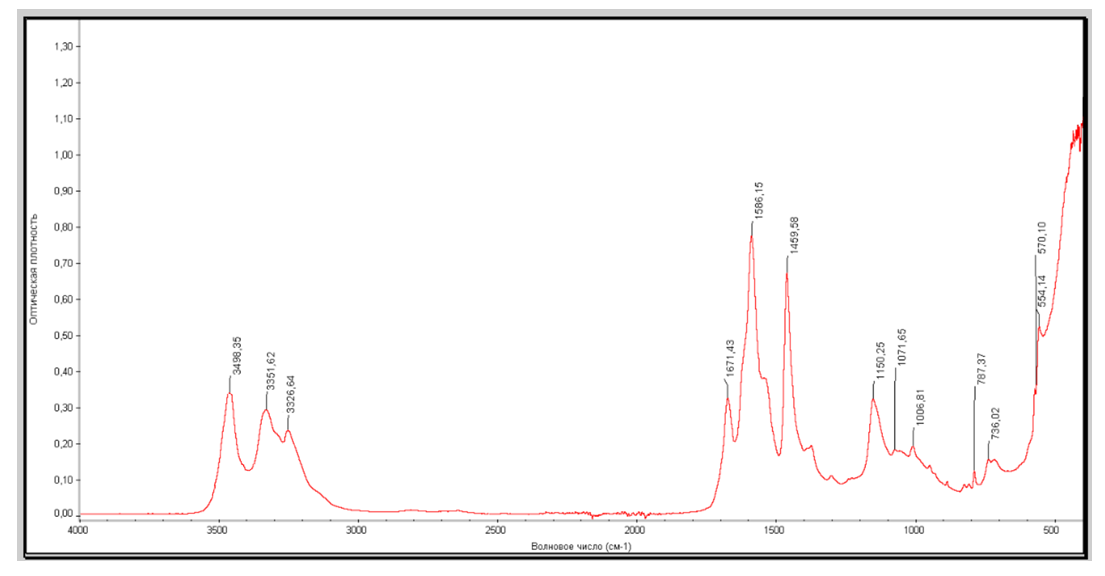 | Figure 1. IR-spectrum of furfurolydenidiurea complex compound |
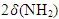 at 3326.64 cm–1; 1671.43 cm–1 and 1585.15 cm–1 absorbances
at 3326.64 cm–1; 1671.43 cm–1 and 1585.15 cm–1 absorbances 
 νas(СОО); νs(СОО) at 1459.58 cm–1;
νas(СОО); νs(СОО) at 1459.58 cm–1; 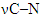 at 1150.25 cm–1; Furan ring at 1006.81 cm–1,
at 1150.25 cm–1; Furan ring at 1006.81 cm–1, 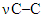 at 787.37 cm–1;
at 787.37 cm–1; 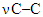 in acetate at 736.02 cm–1 and N–C–N functional groups in deformation vibration at 554.14 cm–1 were identified.As a result of the analysis of the examined complex compound in a scanning electron microscope, its surface appearance was captured. The obtained image clearly showed the morphological structure of the substance. At the same time, the mass fractions of the elements that make it up were determined using the graphical spectrum of the examined substance. In this case, the mass fractions of the elements in the substance were calculated. Figures 2a and 2b show images captured using the device.
in acetate at 736.02 cm–1 and N–C–N functional groups in deformation vibration at 554.14 cm–1 were identified.As a result of the analysis of the examined complex compound in a scanning electron microscope, its surface appearance was captured. The obtained image clearly showed the morphological structure of the substance. At the same time, the mass fractions of the elements that make it up were determined using the graphical spectrum of the examined substance. In this case, the mass fractions of the elements in the substance were calculated. Figures 2a and 2b show images captured using the device.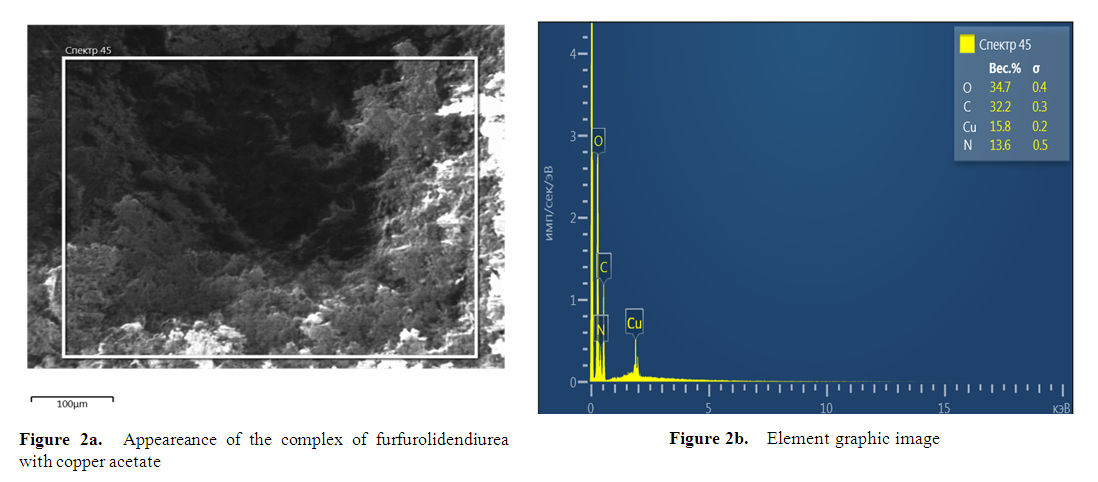 | Figure 2 |
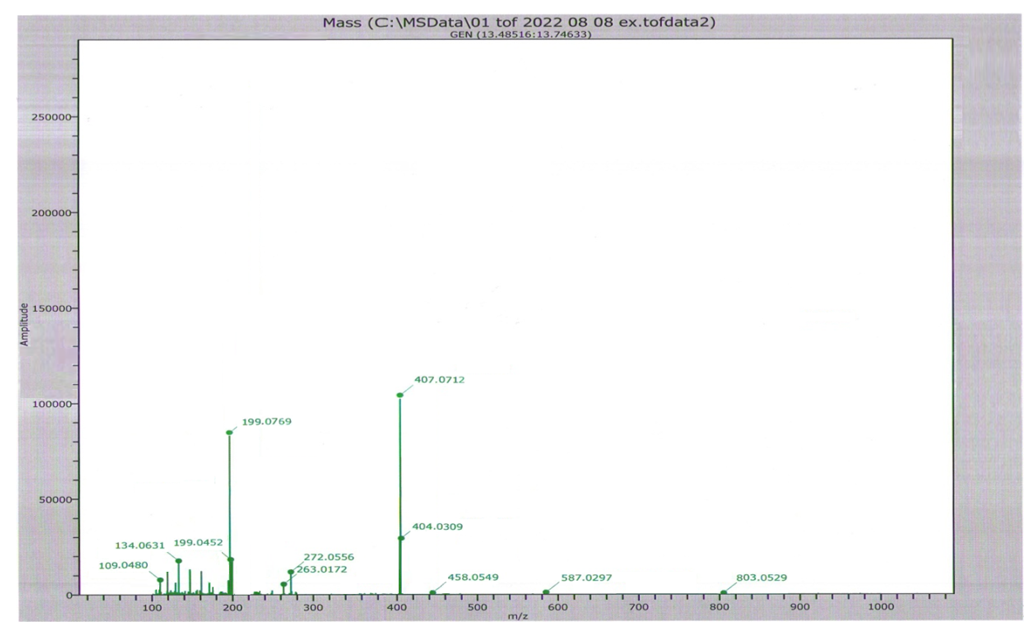 | Figure 3. Mass spectrum of furfurolidendiurea complex |
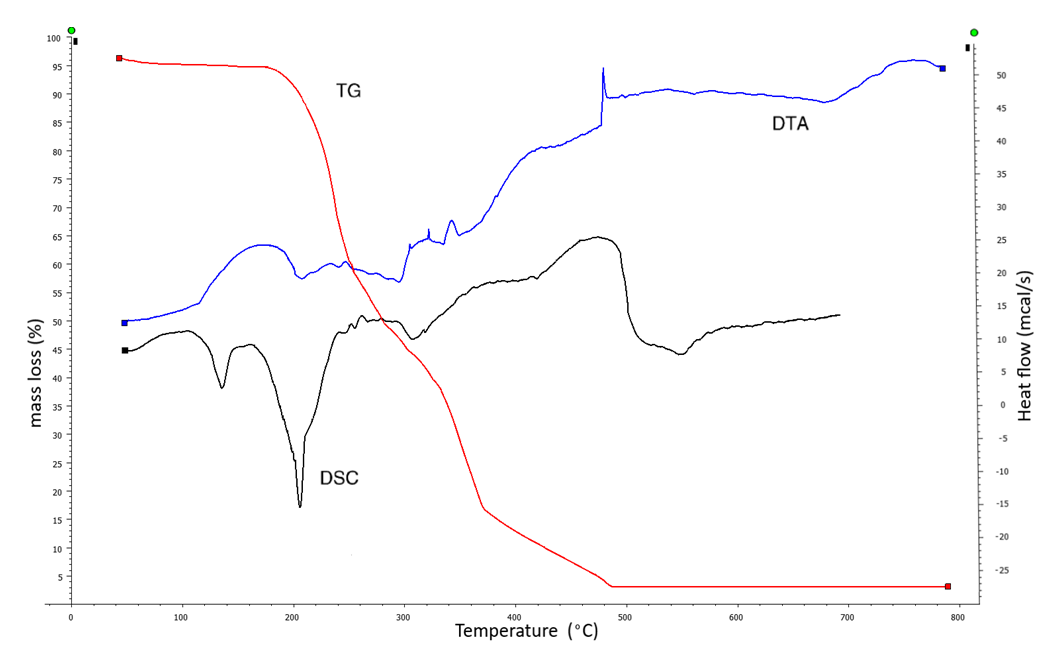 | Figure 4. Derivatogram of the complex formed by furfurolidendiurea with copper acetate |
4. Conclusions
- 1. As a result of IR-spectroscopic and Mass-spectroscopic analysis, it was proved that the compound obtained is a complex formed by furfurolidendiurea with copper acetate.2. The external appearance of the complex crystal was studied with the help of scanning electron microscopic analysis, as a result of the quantitative determination of the elements in it, its compatibility was determined.3. As a result of the analysis, the following complex structure is accepted:
 4. As a result of the thermal analysis of the obtained complex, properties such as heat stability, mass loss, melting were determined.
4. As a result of the thermal analysis of the obtained complex, properties such as heat stability, mass loss, melting were determined. Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML