O. I. Radjabov1, A. S. Turaev1, I. D. Gulmanov2, A. Yu. Otajanov1, L. B. Azimova1
1Institute of Bioorganic Chemistry, Academy of Sciences of the Republic of Uzbekistan
2Tashkent Medical Academy, Uzbekistan
Copyright © 2022 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Abstract
The article presents the results of obtaining dry collagen from cattle skins with the preservation of the original fibrous structure. The physicochemical parameters of the obtained collagen are determined. It has been shown that collagen with a preserved native structure, as well as collagen solutions, must be stored at a temperature of 15-25°C. Based on the obtained collagen, the composition of the injection form was developed, in which the collagen content is a maximum of 18%. Microscopic studies have established that collagen in the injection solution retains its native fibrillar structure. As a result of morphological studies, it was found that the injection solution of collagen does not cause an allergic reaction, does not sensitize the body, does not cause a pronounced inflammatory reaction, and gradually resolves, which indicates the safety of its use.
Keywords:
Collagen, Fibrils, Structure, Injection, Rheological properties
Cite this paper: O. I. Radjabov, A. S. Turaev, I. D. Gulmanov, A. Yu. Otajanov, L. B. Azimova, Obtaining Collagen and Morphological Studies of Injection Solution on Its Basis, International Journal of Materials and Chemistry, Vol. 12 No. 3, 2022, pp. 39-43. doi: 10.5923/j.ijmc.20221203.01.
1. Introduction
The skin, performing its main function - protecting the body from external influences, is also involved in the respiratory, thermoregulatory, excretory, immune and receptor functions, and water-salt metabolism of the body. Age-related changes are accompanied by a decrease in the protective properties of the skin. Skin aging is characterized by thinning, a decrease in elasticity and firmness, impaired collagen homeostasis, and the presence of fine superficial wrinkles [1,2].The safest preparations for injection contour plastics are recognized as materials that include skin components: collagen or hyaluronic acid [3,4]. Collagen is the main structural component of connective tissue and makes up the bulk of the dermis. It has a characteristic amino acid composition and a unique spatial arrangement of polypeptide chains. Unlike other proteins, collagen contains a large amount of the amino acids glycine, proline, hydroxyproline, lysine, and oxylysine, traces of tyrosine and methionine, and cysteine and tryptophan are completely absent [5]. Collagen injections into wrinkles, used since the early 1980s for facial correction, replenish the collagen matrix and promote external rejuvenation. The effect lasts from several weeks to several months [6]. Collagen can accelerate wound healing, enhance platelet adhesion, and induce hemostasis, which, in the absence of antigenicity, has led to its widespread use in reconstructive surgery [7].Collagen-based microimplants have good reason to be considered an ideal material for reconstructive purposes, as they have high biocompatibility with human tissues. Regardless of the field of application, the key to the high clinical effectiveness of collagen-containing preparations and materials is the use of collagen with a preserved native spatial structure in the form of a triple helix. It is structured collagen that can act as a matrix for successful regeneration. In addition, bovine collagen preparations are considered an affordable and least expensive treatment option. All variants of dermal fillers in this group contain 95% bovine type I collagen and 5% type III. However, since bovine collagen protein is different from human, allergy is possible - the risk is thought to be 3% [8]. Therefore, it undergoes special chemical treatment. The technological chain should be designed in such a way as to completely exclude the influence of factors that cause protein denaturation.The purpose of this work is to obtain dry collagen with preservation of the native structure and morphological studies of the skin response to collagen injection with intradermal injection.
2. Materials and Methods
The object of the study was collagen isolated from the skins of cattle under laboratory conditions.
2.1. Getting Collagen
The splits were crushed into pieces no larger than 10×10 mm and washed. Next, alkaline-salt hydrolysis was carried out, containing 10% NaOH and 80–100 g/l Na2SO4, for 60 h. In the next stage, the hides were subjected to buffer extraction with citrate buffer at pH=4.0-4.2 for 24 hours. After the time of the buffer extraction process, the product was washed to a neutral medium. Next, a second alkaline-salt hydrolysis was carried out for 2 hours to obtain highly purified collagen from impurities. After hydrolysis, it was neutralized with a 3% solution of boric acid in a neutral medium and washed with water. The result was a neutral water mass of collagen. For dehydration, the resulting collagen mass was placed in an acetone bath with constant stirring, where it is broken into small pieces under the action of a stirrer and dehydrated. After 4-5 hours, the collagen was removed from the bath and dried by vacuum distillation of acetone. As a result, dry collagen was obtained in the form of amorphous, loose pieces with a fibrous structure, of various shapes and sizes [9].
2.2. Studies of the Rheological Properties
Studies of the rheological properties of a 2% aqueous solution of collagen were carried out on a rotational viscometer “Reotest-2” (Germany) with a working unit of coaxial cylinders in the range of stresses (1.6 - 3∙103) Pa and shear rates (0.2 - 1.3 ∙103) s-1 at temperatures of 15, 20, 25, 30°C [10-11].
2.3. Microscopic Examination
Microscopic examination was performed using an optical microscope LEICA ICC50 (Germany) with a 10 × 10/0.22 objective. To do this, a small number of test substances (at least 5 mg) were placed on a microscope slide. Then the slide was mounted on a microscope stand, the focus of the microscope was adjusted until a clear image was obtained, and a picture was taken using a digital camera.
2.4. Morphological Experiments
Morphological experiments were carried out on 24 male rats weighing 150-200 g under aseptic conditions in the operating unit of the Department of Operative Surgery and Topographic Anatomy of the Tashkent Medical Academy. The injection site (the inner surface of the thigh and back) was shaved and treated twice with alcohol. Before injection, the collagen solution was heated in a bath with warm water at a temperature of 25°C and drawn into syringes of 0.04 ml. Collagen was injected intradermally by the tunnel method. The animals were kept under normal vivarium conditions. For morphological studies, skin pieces in the injection area were taken on days 1, 2, 3, 5, 10, 15, and 30 after the injection of collagen solution. To do this, under ether anesthesia, the marked area of the skin was excised, and the wound was sutured with interrupted sutures. Pieces of tissue were processed by general histological methods and stained with hematoxylin and eosin, and according to van Gieson.
3. Results and Their Discussion
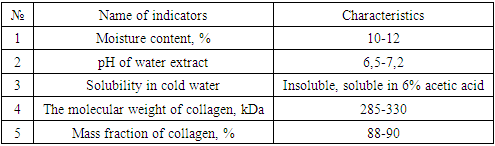
Cattle skin and a chemical method for isolating collagen from hides were used as a raw material for collagen production, which consists in sequentially treating split leather with an alkaline-salt solution and buffer extraction.As a result, dry collagen was obtained with the preservation of the original fibrous structure, convenient for storage and use, in the form of loose and amorphous particles of various sizes and shapes. Physico-chemical parameters of the obtained collagen are presented in the table.Table 1. Dry collagen values
 |
| |
|
To determine the stability of the obtained collagen in solution, we studied the rheological properties of 2% aqueous solutions of collagen in the temperature range of 15-30°C. Information is obtained about the change in the structure under the action of external forces. It is shown that concentrated aqueous solutions of collagen are characterized by an abnormal non-Newtonian viscous flow, which is characteristic of pseudoplastic.With an increase in the temperature of the studied systems from 25 to 30°C, a conformational change in the structure of collagen helices occurs, accompanied by a “helix-coil” transition, as a result of which the supramolecular structure of collagen solutions also changes, the viscosity and structural parameters drop sharply (Fig. 1).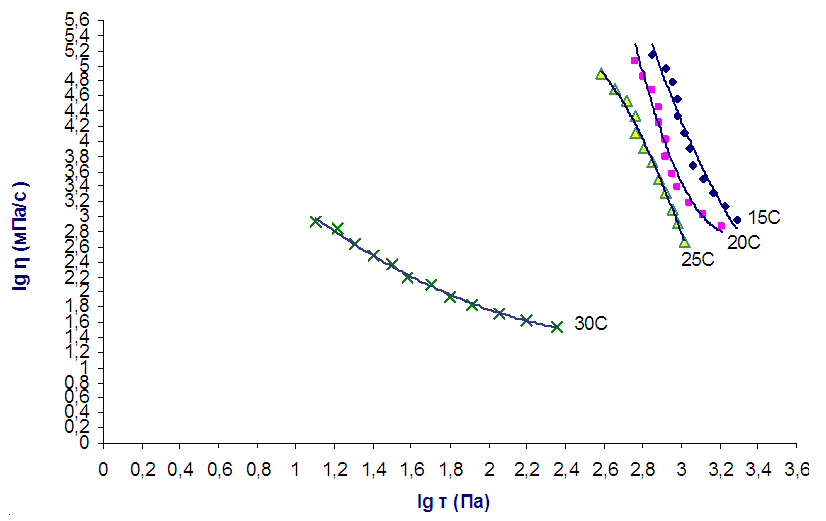 | Figure 1. Flow curves of 2% aqueous solutions of collagen at different temperatures |
As can be seen from Fig. 1, at a temperature of 15-25°C, there is a slight change in viscosity, and therefore in the structure of solutions. When collagen solutions are heated to 30°C and above, a sharp irreversible decrease in viscosity is observed, and a change in the structure of the collagen molecule, i.e., denaturation occurs.Based on the data obtained, it can be concluded that collagen with a preserved native structure, as well as collagen solutions, must be stored at a temperature of 15-25°C. At a solution temperature above 25°C, self-assembly of collagen occurs over time.To obtain an injectable form of collagen, the injectable preparation "Vitreous body" was chosen as a solvent, which contains hyaluronic acid, which is part of human skin, blood, and joints. It is a powerful moisturizer. The combination of collagen with hyaluronic acid enhances the main effect of collagen on recreating the required volume and stimulates the effect of injectable collagen on the development of its connective tissue. The maximum content of collagen in the developed injectable form is 18%. A further increase in the concentration of collagen is impractical due to the high viscosity of the solution. Collagen dissolves well, forming a viscous transparent solution, which is well squeezed at a temperature of 25°C through a needle used for injection with a cross-sectional diameter of 27G.To confirm the preservation of the fibrillar structure of collagen dissolved in the vitreous body, microscopic studies of the collagen solution were carried out using an optical microscope at a magnification of 100 times.Fig. 2 shows that the collagen dissolved in the vitreous retains its native fibrillar structure. Further, morphological studies were carried out on the reaction of the skin to collagen injection with intradermal injection for 1, 2, 3, 5, 10, 15, and 30 days after the introduction of a 10% injection solution of collagen in the vitreous body. The histological features of the dermis and hypodermis at the site of collagen injection and in the intact area were assessed visually by the state of cells and collagen fibers.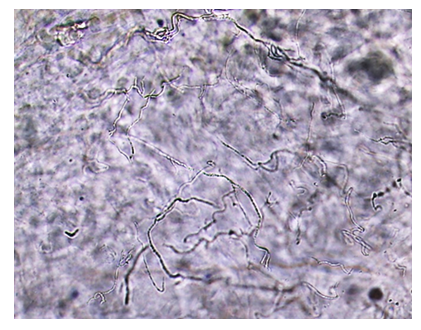 | Figure 2. Microscopic image of collagen dissolved in the vitreous body, with a magnification of 100 times |
On the 1st day of the experiment, the epidermis in the intact zone consists of stratified squamous keratinized epithelium. Swelling around individual epithelial cells. The basement membrane is loosened. Under the epidermis is a thin layer of loose connective tissue. The dermis and hypodermis contain collagen fibers with single fibroblasts, as well as small blood vessels with an enlarged lumen. The epidermis closer to the area of сollagen injection becomes thinner, becomes three-layered with the presence of pericellular edema; and then two-layer with elongated nuclei. In patches, the dermis consists of loose connective tissue. The hypodermis is edematous with the presence of focal leukocyte infiltrate, mainly segmented cells. Here you can see sharply dilated and blood-filled vessels. Collagen fibers are swollen (swollen). Visible blood vessels are dilated and filled with blood. When stained according to van Gieson in the intact zone, the dermis and hypodermis consist of parallel collagen fibers stained bright red. In the area of collagen injection, the dermis is edematous and the connective tissue fibers have a different direction. In the hypodermis, swelling is pronounced, and small foci of round cell infiltration are visible. The vessels are dilated and filled with blood. Some of the collagen fibers are bright red, others are slightly pink.3 days after the injection of collagen, when staining the micropreparation with hematoxylin and eosin, the epithelium of the epidermis is multi-row flat keratinizing, its thickness is different, in the area of collagen injection it is two-three-row. In the dermis, collagen fibers have a parallel arrangement, the edema is moderately pronounced, and individual collagen fibers are clearly visible. Visible blood vessels are dilated. The dermis in the intact zone consists of loose connective tissue with single cellular elements. Hypodermis: moderate swelling is determined on the border with the dermis, with moderately pronounced infiltration along the collagen fibers with the presence of fibroblasts, histiocytes, single leukocytes, and lymphocytes. The process of collagen formation is noted. The hypodermis itself consists of parallel collagen fibers. When staining preparations according to van Gieson, the dermis and hypodermis are represented by mature collagen fibers, stained bright red, at the border of the dermis and hypodermis there are fibroblasts, mature and emerging collagen fibers (bright color - mature; pinkish - maturing).On the 5th day from the beginning of the experiment, the epidermis consists of two or three layers of cells. The dermis and hypodermis in the area of collagen injection consist of tightly adjacent parallel layers of collagen fibers with moderate swelling and single cells of connective tissue (Fig. 3a). 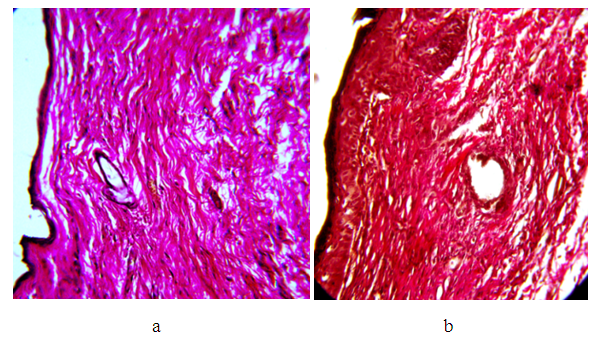 | Figure 3. Introduction zone. Hematoxylin and eosin. Magnification x200: a) Collagen fibers tightly adjacent to each other with moderate swelling. 5th day; b) Densely arranged collagen fibers. 15th day |
Next to this zone, the dermis consists of young formed connective tissue. Collagen fibers are located parallel to the epidermis with the presence of connective tissue cells. Especially a lot of cells under the epidermis. The closer to the intact zone, a moderately pronounced stimulation of collagen fibers is noted in the dermis and hypodermis. On the preparations, areas are visible where the remains of the injected collagen are preserved, around which the formed connective tissue is noted. When stained according to van Gieson, brightly colored collagen fibers in the dermis are located tightly to each other with the presence of single connective tissue cells. In the zone of collagen injection, stimulation in the dermis is noted, and in the adjacent intact zone, connective tissue cells and collagen fibers, colored pink, are visible. The hypodermis has collagen fibers that are loosely arranged, but colored bright red. Fibroblasts and collagen fibers are visible in the hypodermis.On the 10th day of the experiment, the multi-layered, flat, keratinized epidermis consists of two to three layers of cells. In the dermis, collagen fibers with moderate swelling are detected, they are colored bright red, located tightly to each other, and connective tissue cells are noted around them. The hypodermis (Fig. 4) contains many loose collagen fibers with intense staining, as well as fibroblasts. Next to this zone in the dermis and hypodermis, a young developing connective tissue with the presence of connective tissue cells is visible. Under the hypodermis, unresolved collagen is visible, which is homogeneously colored purple, and around it there is also swelling. When stained according to van Gieson in the area of collagen injection in the middle part of the dermis, collagen fibers are colored bright red, located tightly to each other.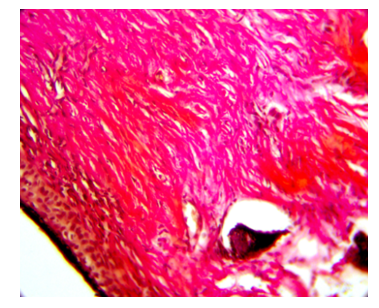 | Figure 4. The area of connective tissue in the hypodermis. Collagen fibers with intense van Gieson staining on the 10th day, x200 magnification |
On the 15th day from the beginning of the experiment, the epidermis consists of numerous squamous keratinized epithelium. The dermis and hypodermis consist of formed connective tissue, and collagen fibers lie tightly (Fig. 3b). Fibroblasts and collagen fibers form a network between mature collagen fibers in the dermis itself. Next to this zone in the dermis and hypodermis, the connective tissue is in a state of transition of loose tissue into a formed one, that is, the development of collagen fibers and a large number of connective tissue cells take place. When stained according to van Gieson in the area of collagen injection, the dermis consists of dense collagen fibers, stained bright red. In the intact zone, collagen fibers are colored red, which indicates the stimulating effect of the injected collagen (increased collagenogenesis).After 30 days, the skin had a normal structure, the dermis was represented by collagen and elastic fibers, no changes in the structure of the epidermis were noted.Based on the studies conducted on the study of the skin reaction to intradermal injection of collagen, it can be concluded that the injection solution of collagen is included in metabolic reactions, and stimulates metabolic processes in the dermis, starting from the first day. Subsequently, on the 5th, 10th, and 15th days there is an increase in metabolic processes, which is manifested by an increase in collagenogenesis, which is confirmed by a bright coloration according to van Gieson of collagen fibers. During these periods, there is a gradual decrease in swelling of the skin tissues. On the 30th day of the experiment, changes in the dermis and hypodermis are insignificant: parallel and densely arranged collagen fibers, fibroblasts, and the formation of young connective tissue with many cells are noted. Subsequently, there is a gradual transition of young loose connective tissue into a formed one, and active fibroblasts are noted.Consequently, the injection solution of collagen does not cause an allergic reaction, does not sensitize the body, does not cause a pronounced inflammatory reaction, and is gradually absorbed. The reaction of tissues to collagen is manifested in the form of moderate swelling, increased metabolic processes, increased collagenogenesis, and activation of fibroblasts and other cells in the hypodermis.
4. Conclusions
Morphological studies have shown that the reaction of the skin to collagen is satisfactory, intradermally injected collagen does not have sensitizing properties and does not cause allergic and inflammatory reactions. Collagen remains in the injection zone during the first day, subsequently, it is resorbed and stimulates collagen formation in the dermis and hypodermis, which is expressed in histological preparations by good staining of collagen fibers according to the van Gieson method, as well as activation of fibroblasts in the connective tissue. The results of the studies carried out testify to the safety of the use of an injection solution of collagen in medicine to increase the volume and compensate for the deficiency of soft tissues and skin defects.
References
| [1] | B. Hertel, Molecular and cellular mechanisms of natural aging and photoaging (stress factors, protective mechanism). Moscow: Cosmetics and medicine, 2000, рр. 5–17. |
| [2] | J. Varani, L. Schuger, M. Dame et al., Reduced fibroblast interaction with intact collagen as a mechanism for depressed collagen synthesis in photoaged skin, J. Invest. Dermatol. 122, 2004, 1471–1479. |
| [3] | I. Danischuk, E. Laputin, Contouring with microimplants. Does an ideal material exist. Moscow: Cosmetics and Medicine, 2001, pp. 63-69. |
| [4] | G.A. Gorbunov, Preparations for injection contour correction of the face. Natural pharmacology and cosmetology. Moscow, 2004, pp.14-15. |
| [5] | E.V. Parfenova, T.M. Ikoev, O.V. Matiytso, The direction of the use of collagen in the technology of cosmetics, Russian Journal of Skin and Venereal Diseases. 2, 2000, 65-67. |
| [6] | R. Glogau, R. Narins, R. Weiss, Advances in cosmetic procedures. Fall Clinical Dermatology, Conference Supplement Proceedings. Supplement to skin and aging. 2004, 20–27. |
| [7] | T.I. Nikolaeva, P.V. Shekhovtsov, Collagen hydrolysates in the prevention and treatment of joint diseases Fundamental Research. 12, 2014, 524 – 528. |
| [8] | M. Alam, H. Gladstone, E.M. Kramer et al., ASDS Guidelines of Care: Injectable Fillers. Dermatologic Surgery, 34(1), 2008, 115-148. doi: org/10.1111/j.1524-4725.2008.34253.x. |
| [9] | O.I. Radjabov, T. Gulyamov, A.S. Turaev, A.Yu. Atazhanov, D.A. Buriev, Study of the physicochemical properties of dry collagen, Universum Journal. 10-1 (76), 2020, 29-31. |
| [10] | R.V. Torner, Theoretical foundations of polymer processing. Moscow: Chemistry, 1977. |
| [11] | G.V. Vinogradov, A.Ya. Malkin, Rheology of Polymers. Moscow: Khimiya, 1977. |





 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML
