-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
International Journal of Materials and Chemistry
p-ISSN: 2166-5346 e-ISSN: 2166-5354
2022; 12(2): 32-38
doi:10.5923/j.ijmc.20221202.03
Received: Aug. 12, 2022; Accepted: Aug. 30, 2022; Published: Sep. 23, 2022

The Composition and Thermodynamic Properties of Pyrolytic Carbon Black
Fozilbek Khudoynazarov1, Suvankul Nurmanov2, Yuldosh Yakubov3
1Basic Doctoral Student, National University of Uzbekistan, Tashkent, Uzbekistan
2Doctor of Technical Sciences, Professor, National University of Uzbekistan, Tashkent, Uzbekistan
3Doctor of Chemical Sciences, Institute of General and Inorganic Chemistry, Tashkent, Uzbekistan
Copyright © 2022 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

The secondary product of acetylene production from methane pyrolysis was purified from soluble salts and oxides by acidic, alkaline and thermal treatment of carbon black, processing. Differential heat, isotherm, thermokinetics and entropy of benzene adsorption in acetylene black carbon were studied using the adsorption-calorimetric method at a temperature of 303 K. The mechanism of adsorption of molecules and the type and number of formed ion-molecular complexes were determined. The adsorption isotherm was characterized by a three-dimensional mathematical equation of the volumetric saturation theory of micro-pores from the initial field to the saturation field.
Keywords: Pyrolysis process, Structure, Acid, Acetylene, Solubility, Isotherm, Adsorption heat, Entropy, Thermokinetics, Ion-molecular complexes, Benzene, Adsorption calorimeter
Cite this paper: Fozilbek Khudoynazarov, Suvankul Nurmanov, Yuldosh Yakubov, The Composition and Thermodynamic Properties of Pyrolytic Carbon Black, International Journal of Materials and Chemistry, Vol. 12 No. 2, 2022, pp. 32-38. doi: 10.5923/j.ijmc.20221202.03.
Article Outline
1. Introduction
- The acetylene carbon black occupies a special place among other structures. The carbon black has high electrical conductivity and a secondary structure. Its particles are connected to each other by strong branched chains.Not only is the high electrical conductivity of the carbon black but also its secondary structure is important. In terms of its activity, the acetylene carbon black is close to the P-1250 furnace gas carbon black. Carbon black is produced on an industrial scale and is also produced in large quantities as a by-product in the process of high-temperature processing of carbon sources. Used in the manufacture of rubber, various cables, ebonite, and insulating materials [1]. It is also used in the adsorption treatment of aqueous solutions, in medicine as a drug to prevent poisoning, and as a fuel source. The composition of the carbon black especially its properties varies, and its properties depend on the process of formation of the carbon black, the initial raw material, technological processes and temperature. In addition to carbon, carbon black also contains some other elements, including hydrogen and oxygen, which form a strong bond with the individual atoms of carbon. On average, for every fifteenth atom of carbon, there is one atom of hydrogen. Oxygen is bound to the outer atoms of carbon by strong chemical bonds [2]. Although the properties of the carbon black have been sufficiently studied, the mechanisms of interaction in its application have not been studied. The growing importance of carbon materials in several chemical processes is due to the importance of these materials in their use as adsorbents and catalysts. The surface area, porosity, chemical inertness, and presence of oxygen in carbon materials affect not only the adsorption properties but also the catalytic activity and selectivity of the catalyst [3]. The mechanism of phenol adsorption in microporous carbon adsorbents and non-carbon samples has been studied and shown to be similar to the benzene adsorption mechanism [4-5]. The use of different types of carbon adsorbents in gas chromatography (activated carbon, carbon molecular sieves, graphitized carbon adsorbents, etc.) is analyzed and their general surface properties, methods of preparation, modification, and application are studied.
2. Materials and Methods
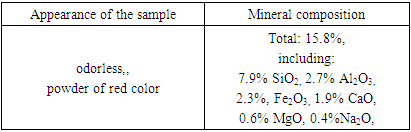
- The secondary product of acetylene extraction from methane pyrolysis is carbon black. It was treated with hydrochloric acid of different concentrations at different time intervals and the solubility of the resulting product was determined. Determination of the composition of samples of raw materials and purified products under acidic conditions was carried out using a scanning electron microscope SEM - EVO MA 10. The literature contains information on the adsorption of benzene in various carbon black, which were obtained by various physicochemical research methods [9]. The adsorption energy and mechanism of benzene as a sorbent in the processed carbon black have not been studied. Adsorption of benzene at a temperature of 303 K was studied in the carbon black (a). Differential heat values of adsorption (kJ) were determined using a high-precision adsorption-calorimeter device [10]. The device is a high vacuum-resistant glass device equipped with a micro burette and symbol transmissions. It consists of an adsorbent ampoule, a measuring part, a storage system, a gas and liquid collector and a vacuum system. The high-precision differential microcalorimeter DAK 1-1A was used in the study. Initially, the sample was heated under vacuum at 10–4 Pa for 10 h, in the closed state at 1023 K. The experiment was performed on an adsorption-calorimetric device [11]. Adsorption heat and isotherm values were calculated at 303 K.
3. Results and Discussion
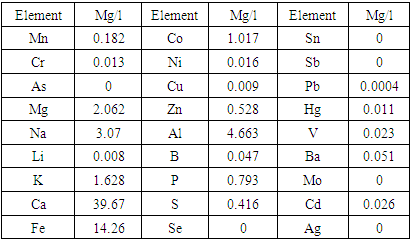
- The composition of acetylene carbon black was analysed for macro- and microelements by optical emission spectrometry. The results of the analysis are presented in Table 1.
|
|
|
|
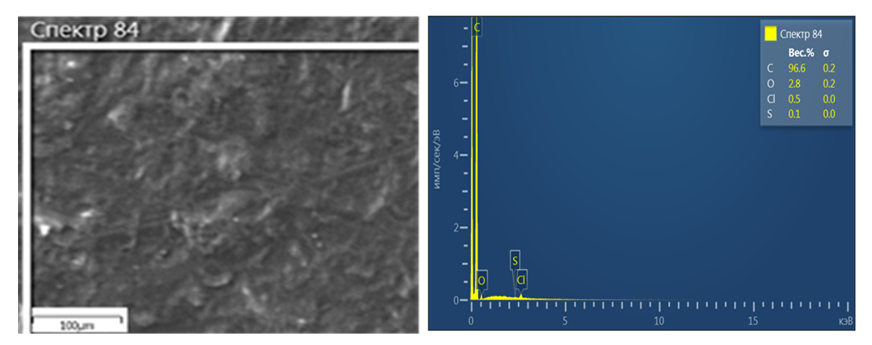 | Figure 1. SEM image and analysis of carbon black sample: a) Image of carbon black processed in 15% acid; b) The elemental composition of the carbon black treated with 15% acid |
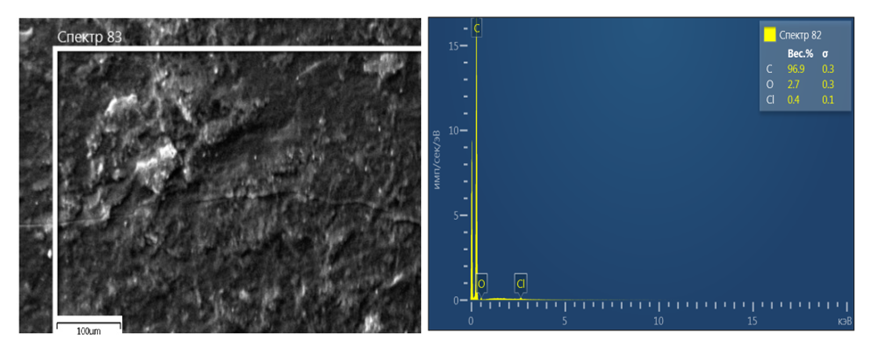 | Figure 2. SEM image and analysis of carbon black sample: a) Image of carbon black processed in 20% acid; b) The elemental composition of the carbon black treated with 20% acid |
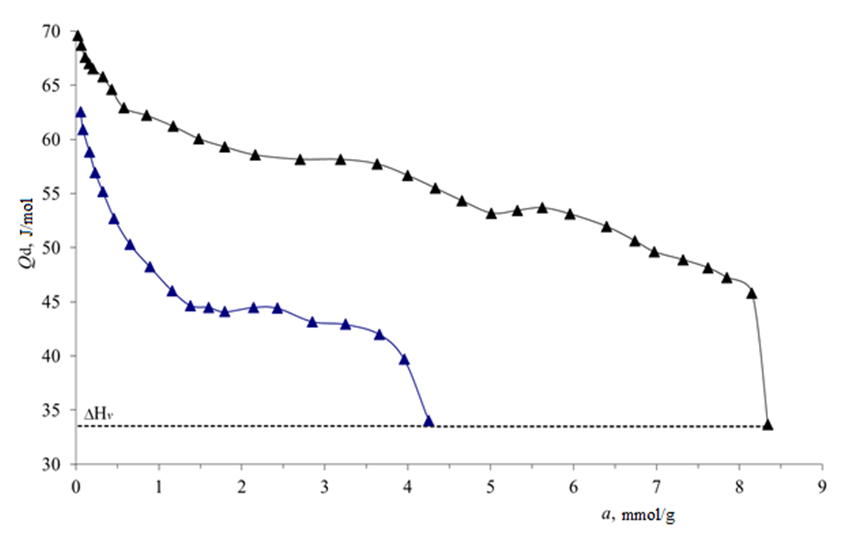 | Figure 3. Differential heat values (Qd) of benzene adsorption are given in  -modified carbon black, -modified carbon black,  -unmodified carbon black samples at 303 K. Barcodes Condensation value of benzene at 303 K -unmodified carbon black samples at 303 K. Barcodes Condensation value of benzene at 303 K |
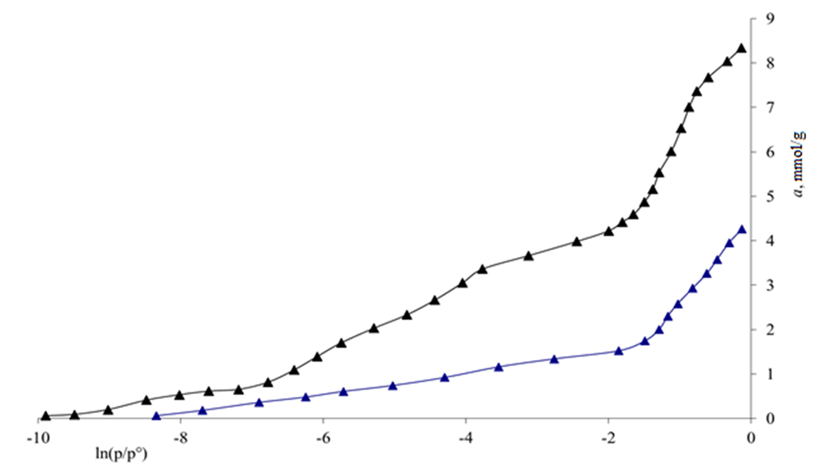 | Figure 4. Adsorption values of benzene vapour adsorption in  -modified carbon black and -modified carbon black and  -unmodified carbon black samples at 303 K are given in ln (P/P0) -unmodified carbon black samples at 303 K are given in ln (P/P0) |
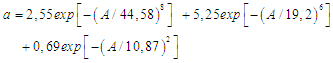 Where a is adsorption (mol/g), A = RTln (P/P) is the work done to bring 1 mole of gas (at pressure P) to the surface in equilibrium in the gas phase (pressure R). The adsorption isotherm of unmodified carbon black benzene is described by the three-dimensional equation of MHTN.
Where a is adsorption (mol/g), A = RTln (P/P) is the work done to bring 1 mole of gas (at pressure P) to the surface in equilibrium in the gas phase (pressure R). The adsorption isotherm of unmodified carbon black benzene is described by the three-dimensional equation of MHTN.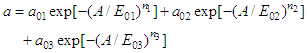 These values are: a01 = 1.8 mmol/g, E01 = 14.1 KJ/mol and n1 = 7; for the second member a02 = 2.1 mmol/g, E02 = 15.26 KJ/mol and n2 = 12; the third member values a03 = 0.28 mmol/g, E02 = 8.99 KJ/mol, and n3 = 3. It can be seen that adsorption in the microstructure is almost non-existent in the secondary structure. The adsorption differential entropy was calculated using the Gibbs-Helmholtz equation using the isotherm and differential heat value. Figure 5 shows that in the initial cases, the unmodified carbon black adsorption values range from 0.043 mmol/g to 4.25 mmol/g, and the entropy values range from -12-19 J/mol · K to -18-0 J/mol · K.
These values are: a01 = 1.8 mmol/g, E01 = 14.1 KJ/mol and n1 = 7; for the second member a02 = 2.1 mmol/g, E02 = 15.26 KJ/mol and n2 = 12; the third member values a03 = 0.28 mmol/g, E02 = 8.99 KJ/mol, and n3 = 3. It can be seen that adsorption in the microstructure is almost non-existent in the secondary structure. The adsorption differential entropy was calculated using the Gibbs-Helmholtz equation using the isotherm and differential heat value. Figure 5 shows that in the initial cases, the unmodified carbon black adsorption values range from 0.043 mmol/g to 4.25 mmol/g, and the entropy values range from -12-19 J/mol · K to -18-0 J/mol · K.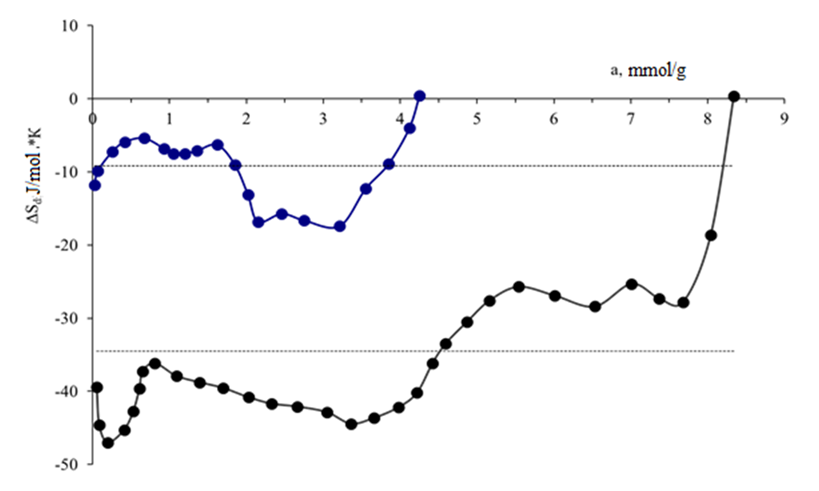 | Figure 5.  - modified at 303 K and - modified at 303 K and  - entropy of adsorption of benzene vapours on unmodified carbon black samples. Barcodes give an average integral entropy value - entropy of adsorption of benzene vapours on unmodified carbon black samples. Barcodes give an average integral entropy value |
 | Figure 6. Kinetics of adsorption of benzene vapours in modified (-) and unmodified carbon black (-) samples at 303 K |
4. Conclusions
- As a result of acid treatment of carbon black, its quality has improved, and the level of sol has been reduced. The effect of hydrochloric acid concentration on the sol of level was determined and the process conditions were optimized. When treated in a 30% solution of hydrochloric acid for 4-5 hours, the sol of the carbon black was reduced from 15.8% to 1.88%.The adsorption heat stepwise and adsorption properties of the systems studied with low saturation were determined. Molecular mechanisms of adsorption of benzene on carbon black, and interrelationships between adsorption-energy characteristics were determined. Adsorption of benzene molecules to the active centres of carbon black has been shown to proceed with the formation of p-complexes. Adsorbed benzene molecules are in a stationary state, i.e., in the solid state entropy. A correlation was observed between the thermodynamic characteristics of the studied systems.Based on the calculated and experimental data, it can be said that the adsorption of benzene in carbon black consists of the localization of benzene molecules with a sandwich-like character in the active centre, in the pores and around it.
ACKNOWLEDGEMENTS
- The authors acknowledge the immense help received from the scholars whose articles are cited and included in references to this manuscript. The authors are also grateful to the authors/ editors/publishers of all those articles, journals and books from where the literature for this article has been reviewed and discussed.In addition, the authors express gratitude to the head of the National University of Uzbekistan and the scientific community of the University for their assistance in carrying out this research work.The authors report no conflicts of interest. The Source of funding is nil.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML