-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
International Journal of Materials and Chemistry
p-ISSN: 2166-5346 e-ISSN: 2166-5354
2022; 12(1): 14-19
doi:10.5923/j.ijmc.20221201.03
Received: Jun. 20, 2022; Accepted: Jul. 9, 2022; Published: Jul. 11, 2022

Synthesis and Study of the Antibacterial Activity of Polyvinylpirrolidone Polymeric Complexes with Levofloxacin
Abrekova N. N., Mahmudov S. D., Beknazarova N. S., Turaboev Sh. M., Sagdullaev B. T.
Institute of Bioorganic Chemistry, Academy of Sciences of the Republic of Uzbekistan
Copyright © 2022 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

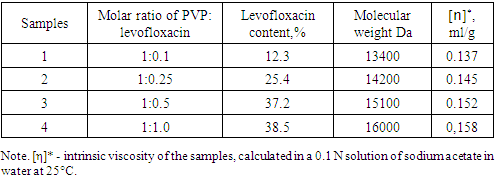
In the present work, chemical immobilization of levofloxacin into macromolecules of a synthetic polymer, polyvinylpyrrolidone, was carried out. By varying the molar ratio of PVP: levofloxacin in the reaction medium, polymer complexes were obtained in which the quantitative content of the drug was 12.3-38.5%. The structure of the synthesized polymer compounds was confirmed by IR and UV spectroscopy, and the molecular characteristics of the reaction products were also calculated. The kinetics of cleavage of levofloxacin from polyvinylpyrrolidone macromolecules at pH 2.0 and 7.4 was studied. According to data the most optimal is the use of an antibacterial complex with a molar ratio of PVP and levofloxacin respectively is 1:0.5.
Keywords: Fluoroquinolones, Levofloxacin, Polyvinylpyrrolidone, Polymer complex, Antibacterial properties
Cite this paper: Abrekova N. N., Mahmudov S. D., Beknazarova N. S., Turaboev Sh. M., Sagdullaev B. T., Synthesis and Study of the Antibacterial Activity of Polyvinylpirrolidone Polymeric Complexes with Levofloxacin, International Journal of Materials and Chemistry, Vol. 12 No. 1, 2022, pp. 14-19. doi: 10.5923/j.ijmc.20221201.03.
Article Outline
1. Introduction
- One of the most practical ways to obtain physiologically active polymers with a pharmacological property, is the chemical immobilization of low molecular weighted biologically active compounds to macromolecules of natural or synthetic polymers. It is known that the modification of low molecular weight drugs with various polymers is carried out in order to improve their therapeutic properties, eliminate existing side effects, increase the duration of their action in the body, ensure targeted transport of the active substance and increase bioavailability [1,2]. Due to a sharp increase in the resistance of pathogens of infectious diseases to low molecular weight antimicrobial drugs used in clinical practice, a search is being intensively aimed at creating antimicrobial macromolecular systems consisting of a polymer matrix and an active substance [3-5]. Mainly, the specific properties of the graft polymer designed according to this principle are determined by the physicochemical characteristics, molecular weight and the type of chemical bond formed between the drug and the macromolecular chain [6,7]. Therefore, by optimizing the final structure and the necessary characteristics of the created antimicrobial polymer, it is possible to obtain new drugs that are devoid of the original disadvantages and have the desired pharmacological properties.Clinical studies have shown that fluoroquinolones are highly effective antibacterial drugs that are very often used in the treatment of infections, including resistant forms of tuberculosis [8-10]. Despite a wide range of antimicrobial activity, the main disadvantages of fluoroquinolone preparations are low bioavailability and the presence of various side effects on the body during long-term use. For example, levofloxacin, an antibacterial drug that is part of the third-generation fluoroquinolones. Levofloxacin is often prescribed in clinical practice because it is highly effective against most strains of bacterial pathogens responsible for respiratory, urinary, gastrointestinal and abdominal infections. However, the half-life of levofloxacin is 6-8 hours. In this connection, to create the necessary therapeutic concentration in an infected organism it is necessary to increase the daily dosage of levofloxacin. And as a result, there is a clinical detected cardiotoxicity (prolongation of the QT interval), nephrotialysis, hyperkalemia, allergic reactions, etc.Based on the above-described relevance of scientific and practical problems, in this work we carried out the chemical modification of levofloxacin with a synthetic hydrophilic polymer polyvinylpyrrolidone (PVP). The choice of PVP as a polymer matrix for levofloxacin can be substantiated by the fact that this polymer is highly soluble in water, has unique physicochemical properties, is nontoxic, and contains reactive functional groups in its structure that easily bind drugs by complex formation [10-12].The objective of this work was to obtain and study the biological activity of polymer complexes of levofloxacin with polyvinylpyrrolidone, in which the antibacterial agent is associated with hydrophilic polymer macromolecules by a hydrolyzable type of chemical bond.
2. Materials and Methods
- To carry out the chemical modification of levofloxacin with hydrophilic polymers, we used polyvinylpyrrolidone with a molecular weight of 12000 Da, purified by extraction with diethyl ether.
2.1. Chemical Modification of Levofloxacin with Polyvinylpyrrolidone
- 0.0135 mole (1.5 g) of PVP was dissolved at room temperature in 50 ml of water until a clear solution was formed. After complete dissolution of the polymer with vigorous stirring, 0.00135-0.0067 mole (0.49-2.44 g) of levofloxacin was added. The reaction solution was stirred for 2 hours and t=20°C. The resulting clear solution was then dialyzed against distilled water for 24 hours using dialysis bags with a protein cutoff of 5000 Da. The final products were isolated by sublimation of water from a pre-frozen aqueous solution.
2.2. Study of the Structure and Molecular Characteristics of Polymer Complexes of Levofloxacin with Polyvinylpyrrolidone
- UV spectra of aqueous solutions of the synthesized samples were taken on a “UV 1280” spectrophotometer (Shimadzu, Japan) in the range λ=180-400 nm. IR spectra were recorded on a Vector-22 spectrometer in the wavelength range 400-4000 cm-1 in KBr tablets (3 mg sample/300 mg KBr). The molecular weight characteristics of the synthesized derivatives were determined by high performance gel permeation chromatography on an Agilent 1260 Infiniti liquid chromatograph. The analysis process was monitored using a refractometric detector using a PL Aquagel OH Mixed chromatographic column 300 mm long and 7.5 mm in inner diameter (Waters, USA). A 0.1 N aqueous solution of sodium nitrate was used as the eluent. The volume flow rate of the eluent was 0.8 ml/min.
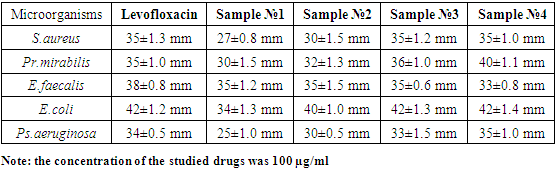
2.3. Determination of Antibacterial Properties of Polymer Complexes of Levofloxacin with Polyvinylpyrrolidone in vitro
- We chose the following microorganisms as test cultures for determining the antimicrobial activity of levofloxacin polymer complexes: S.aureus, Pr.mirabilis, E.faecalis, E.coli, and Ps.aeruginosa. The antimicrobial effect of the synthesized compounds was studied at a concentration of 100 µg/ml by the agar diffusion method, based on the ability of the studied substances to diffuse into the nutrient medium on which the selected test cultures are sown and to suppress the growth of microorganisms (mm). The diameter of microbial growth inhibition zones less than 10 mm was evaluated as the absence of antimicrobial activity, 10-15 mm - weak activity, 15-20 mm - moderately pronounced activity, more than 20 mm - pronounced activity. Each sample was tested in three parallel experiments. The obtained data were statistically processed taking into account Student's criterion [13].
2.4. Study of the Release of Levofloxacin from PVP Macromolecules
- The release rate of levofloxacin from polymeric complexes was assessed by dialysis through a semipermeable membrane with a protein transmission limit of 3000 Da. The hydrolysis of polymer complexes was studied in physiological solution with pH 2.0 and 7.4 at a temperature of 25°C using equipment for the study of equilibrium dialysis. The selected polymer complex dissolved in 5 ml of physiological solution was placed in the left cell of the setup. The optical density of the prepared solution λmax=294 nm was 2.23. Then, 5 ml of physiological solution was placed in the right cell. At certain intervals, samples of 0.5 ml of the solution were taken from the right cell and diluted 10 times, and the amount of levofloxacin that passed through the cellophane membrane of the right cell was determined spectrophotometrically at λmax=294 nm in the resulting solution. The degree of hydrolysis (S,%) of the polymer complex was calculated as the ratio of D294 in the right cell at time τ to the initial value of D294 in the left cell and expressed in % [14].
3. Results and Their Discussion
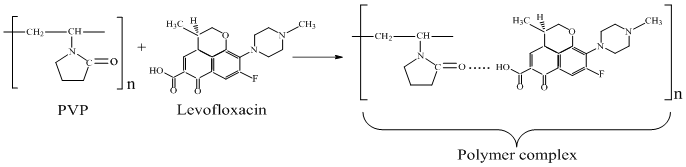
- The high reactivity of polyvinylpyrrolidone is associated primarily with its ability to form complex compounds (due to the presence of >N-C=O groups in the macromolecule) with substances containing -COOH groups in the structure. Therefore, the chemical binding of levofloxacin to PVP functional groups will proceed according to the following scheme:
 To determine the limiting number of lefoloxacin molecules that can bind to PVP macromolecules, we carried out the reaction at different molar ratios of the starting reagents (Table 1). The molecular characteristics of the obtained samples were also found.
To determine the limiting number of lefoloxacin molecules that can bind to PVP macromolecules, we carried out the reaction at different molar ratios of the starting reagents (Table 1). The molecular characteristics of the obtained samples were also found.
|
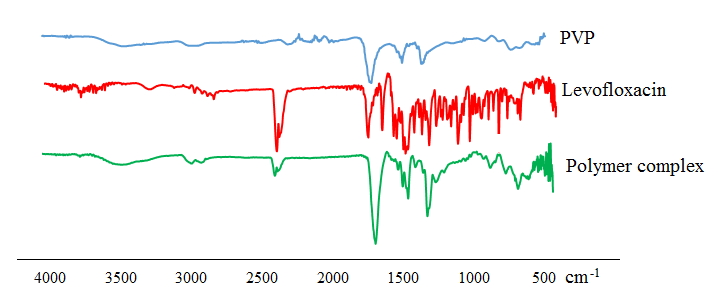 | Figure 1. IR spectra of polyvinylpyrrolidone, levofloxacin and polymer complex-3 |
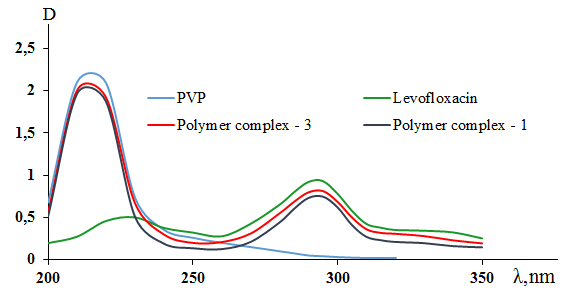 | Figure 2. UV spectra of povinylpyrrolidone, levofloxacin, and synthesized polymer complexes 1 and 3 |
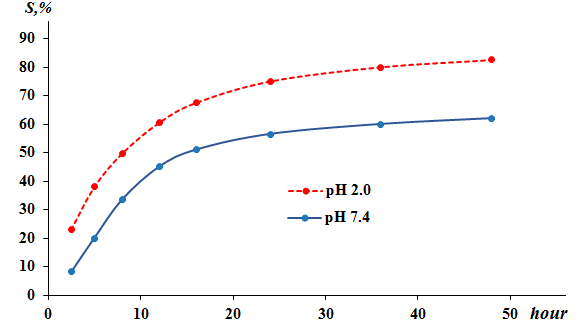 | Figure 3. Kinetics of Levofloxacin Release from the Polymer Complex at pH 2.0 and 7.4 (Levofloxacin Content in the Polymer Complex is 37.2%) |
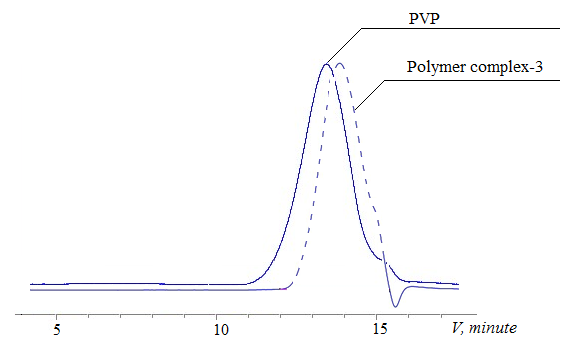 | Figure 4. Gel chromatograms of PVP and polymer complex-3 with a quantitative content of levofloxacin 37.2% |
|
4. Conclusions
- Thus, by complexation of the >N–C=O groups of PVP and -COOH groups of levofloxacin, water-soluble polymer complexes with different drug content of 12.3-38.5% were obtained for the first time. By carrying out the release kinetics of the polymer complex, it was proved that the degree of hydrolysis of levofloxacin from PVP macromolecules at pH 2.0 after 24 and 48 hours is -74 and 82.5%, and at pH 7.4 - 53 and 62%. Based on the comparative studies conducted, it can be concluded that the preparation of an antibacterial complex with a molar ratio of PVP: levofloxacin = 1:0.5 according to the conditions of synthesis, therapeutic activity, technological and practical reproducibility is the most optimal.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML