-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
International Journal of Materials and Chemistry
p-ISSN: 2166-5346 e-ISSN: 2166-5354
2022; 12(1): 10-13
doi:10.5923/j.ijmc.20221201.02
Received: Feb. 3, 2022; Accepted: Feb. 23, 2022; Published: Feb. 28, 2022

Quantitative Analysis of Polycyclic Compounds in Heavy Pyrolysis Oil by High-Efficiency Liquid Chromatography
F. Z. Burkhev, H. H. Kushiev, T. A. Djuraev
Gulistan State University, Gulistan, Uzbekistan
Copyright © 2022 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

The article presents heavy pyrolysis oil (black, pungent-smelling poisonous liquid) formed as a result of pyrolysis of natural gas and containing polycyclic aromatic ring hydrocarbons (PAH) for the growth and development of plants and for physiologically active substances important in industrial production. indene and naphthalene were thermally fractionated. The obtained substances were quantified on Prominence-i LC-2030C 3D Plus (Shimadzu) using standard (Sigma Aldrich) samples by High Efficiency Liquid Chromatography (HELC) method. According to the results of the study, pyrolysis oil contains 9.21% indene, and 23 - 25% naphthalene, and due to the crystalline structure of the separated naphthalene, as a result of re-fractionation obtained pure naphthalene with a purity of 98.4%. The fractionation of pyrolysis oil, which is an additional product, has created a raw material base for the production of new types of import-substituting products.
Keywords: HELC, Chromatography, Pyrolysis, Heavy pyrolysis oil, Inden, Naphthalene, Stationary phase
Cite this paper: F. Z. Burkhev, H. H. Kushiev, T. A. Djuraev, Quantitative Analysis of Polycyclic Compounds in Heavy Pyrolysis Oil by High-Efficiency Liquid Chromatography, International Journal of Materials and Chemistry, Vol. 12 No. 1, 2022, pp. 10-13. doi: 10.5923/j.ijmc.20221201.02.
1. Introduction
- Most products, such as ethylene and propylene, which are the basis for high polymer compounds, are obtained by pyrolysis of hydrocarbons (the process of decomposing many organic and inorganic compounds into simple substances under air at high temperatures, sometimes in the presence of catalysts). Pyrolysis of hydrocarbons also undergoes fusion reactions, resulting in the formation of aromatic and unsaturated ring hydrocarbons with high molecular weight as a secondary product [1].
2. The Main Findings and Results
- The formation of an unknown polymer from the residue and the lack of methods for the detection of polymer monomers in the pyrolysis oil and the lack of separation technology hamper the use of raw materials. Therefore, one of the most pressing problems today is the introduction of a system for the processing of heavy pyrolysis oil and the separation of substances containing functional groups, which is formed on the basis of production activities of this enterprise.In the following years, high-efficiency liquid chromatography (HPLC) began to be used for the separation and detection of polyaromatic hydrocarbons (PAH). According to Schmidt's research [2], C18 columns selected as a stationary phase are widely used in the HELC method for PAH separation. Liquid chromatography (LC) combined with mass spectrometry (HPLC-MS) is also noted as one of the most important analytical methods in the last decade [6]. It has relevant advantages in the Manufacturing and Pharmaceutical industries and also plays an important role in environmental analysis [3,4]. It is noted that the pyrolysis process can also produce aromatic hydrocarbons Inden and naphthalene [4,7].
 Inden - C9H8 occurs naturally in coal tar resin fractions boiling around 185°C. This fraction can be obtained by heating with sodium and precipitating the solid “sodium-indene”. Sodium is converted to indene by water [5,9].Naphthalene is an aromatic hydrocarbon composed of 2 condensed benzene rings containing C10H8. A white, plate-like crystal with a distinctive odor. 128.2g / mol, Tsuy = 80.3°, Tqay = 218°. Toxic, density 1025 kg / m3. Less soluble in water. Soluble in alcohol, ether, benzene. Coal is extracted from dyogoti [8,10].
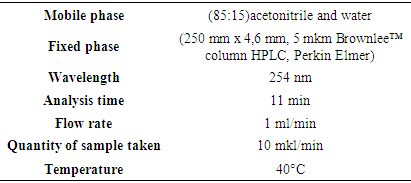
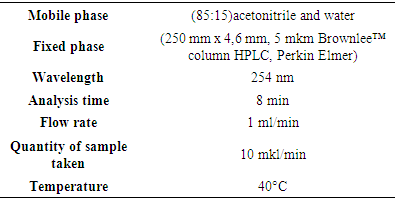
Inden - C9H8 occurs naturally in coal tar resin fractions boiling around 185°C. This fraction can be obtained by heating with sodium and precipitating the solid “sodium-indene”. Sodium is converted to indene by water [5,9].Naphthalene is an aromatic hydrocarbon composed of 2 condensed benzene rings containing C10H8. A white, plate-like crystal with a distinctive odor. 128.2g / mol, Tsuy = 80.3°, Tqay = 218°. Toxic, density 1025 kg / m3. Less soluble in water. Soluble in alcohol, ether, benzene. Coal is extracted from dyogoti [8,10]. In this study, research was conducted to determine the presence of these compounds in pyrolysis oil.In our research, the object of research was a secondary product based on gas processing - heavy pyrolysis oil by the joint venture “Uz-Kor Gas” LLC. Pyrolysis of gases produces pyrolysis oil as a by-product as a raw material for polymer products.The formation of aromatic hydrocarbons (SVUF - MS method), which are the main components of pyrolysis oil, along with low molecular weight monomers, is theoretically based on the fact that the temperature in the reactor rises to 600-800°C during pyrolysis. The first fraction of pyrolysis, i.e. light fractions up to 210°C, were separated and divided into components such as indene and methyl-indene.During the re-driving of the first fraction, it was found that in addition to indene and methyl-indene, there are additional substances, including naphthalene. The polymerization of the residual substance between light and time was determined. During the study, based on spectroscopic analysis, the monomer composition of the polymer was determined according to the functional groups reflected in the IR spectra. Once the composition of the polymer link was determined, its molecular mass (MM) and degree of polymerization were determined viscometrically.Quantitative analysis of indene and naphthalene in the chemical analysis department of the laboratory “Experimental Biology” on the device Prominence LC-2030C 3D Plus (Shimadzu, Japan) and analytical column (250 mm x 4.6 mm, 5 μm Brownlee ™ HPLC column, Perkin Elmer).For the study, the Uzbek-Korean joint venture “Uz-Kor Gas” received heavy pyrolysis oil, which is produced as a secondary product on the basis of gas processing.As a result of heat treatment (fractional driving), liquids from 165 to 205°C containing inden (Tqay = 181°C) were separated in a selective separation device - deflegmator. Under normal conditions, the aggregate was separated from the liquid in the form of a yellow mixture. Naphthalene (Tqay = 214°C), a white crystalline substance formed on the walls of the refrigerator at a temperature of 215° to 220°C, was isolated. The amount of substances extracted and the degree of purity are related to the processing of the raw material, and the percentage of the product obtained is mainly important for the industry.The HELC method is an excellent analytical method for the quantitative analysis of naphthalene, indene and other organic substances. Solutions for analysis were prepared from the isolated substances for the purpose of analysis by the method of HELC. Solutions of liquid and acetonitrile (CH3CN) in a ratio of 1:99 and pure naphthalene crystals in a ratio of 1: 2000 in acetonitrile (CH3CN) were prepared.Quantitative analysis of indene and naphthalene in the chemical analysis department of the laboratory “Experimental Biology” on the device Prominence LC-2030C 3D Plus (Shimadzu, Japan) and analytical column (250 mm x 4.6 mm, 5 μm Brownlee ™ HPLC column, Perkin Elmer). A 10 μL / min sample solution was added to the column at a gradient flow of 1.0 ml / min of the excitable phase mixture of acetonitrile and water (85:15). The temperature in the column was set to 40°C. A total of 30 min is spent for each sample extract from the mobile phase with multi-stage gradient conditions.PDA diode - matrix absorption detector is used at a wavelength of 254 nm to increase sensitivity and selectivity. The second detector used is the fluorescent detector used in the RF-20Axs monochromator drive and emission wavelength program. This allows the best sensitivity to be achieved using the detector. As a result, it allows the detection of all types of PAUs, including inden and naphthalene.Standard samples of inden and naphthalene for analysis were solutions of different concentrations from Inden (193828 - 1G> 98% HPLC, Sigma Aldrich) and naphthalene (147141 - 25G> 99% HPLC, Sigma Aldrich): 0.05mg / ml 0.1mg / ml and 0.2 mg / ml solutions were prepared and calibrated. During the calibration of the standards, inden and naphthalene were found to have good absorption in the PDA diode-matrix absorption detector. Sample analysis was also performed on this detector. The samples isolated for analysis were analyzed based on the above method.
In this study, research was conducted to determine the presence of these compounds in pyrolysis oil.In our research, the object of research was a secondary product based on gas processing - heavy pyrolysis oil by the joint venture “Uz-Kor Gas” LLC. Pyrolysis of gases produces pyrolysis oil as a by-product as a raw material for polymer products.The formation of aromatic hydrocarbons (SVUF - MS method), which are the main components of pyrolysis oil, along with low molecular weight monomers, is theoretically based on the fact that the temperature in the reactor rises to 600-800°C during pyrolysis. The first fraction of pyrolysis, i.e. light fractions up to 210°C, were separated and divided into components such as indene and methyl-indene.During the re-driving of the first fraction, it was found that in addition to indene and methyl-indene, there are additional substances, including naphthalene. The polymerization of the residual substance between light and time was determined. During the study, based on spectroscopic analysis, the monomer composition of the polymer was determined according to the functional groups reflected in the IR spectra. Once the composition of the polymer link was determined, its molecular mass (MM) and degree of polymerization were determined viscometrically.Quantitative analysis of indene and naphthalene in the chemical analysis department of the laboratory “Experimental Biology” on the device Prominence LC-2030C 3D Plus (Shimadzu, Japan) and analytical column (250 mm x 4.6 mm, 5 μm Brownlee ™ HPLC column, Perkin Elmer).For the study, the Uzbek-Korean joint venture “Uz-Kor Gas” received heavy pyrolysis oil, which is produced as a secondary product on the basis of gas processing.As a result of heat treatment (fractional driving), liquids from 165 to 205°C containing inden (Tqay = 181°C) were separated in a selective separation device - deflegmator. Under normal conditions, the aggregate was separated from the liquid in the form of a yellow mixture. Naphthalene (Tqay = 214°C), a white crystalline substance formed on the walls of the refrigerator at a temperature of 215° to 220°C, was isolated. The amount of substances extracted and the degree of purity are related to the processing of the raw material, and the percentage of the product obtained is mainly important for the industry.The HELC method is an excellent analytical method for the quantitative analysis of naphthalene, indene and other organic substances. Solutions for analysis were prepared from the isolated substances for the purpose of analysis by the method of HELC. Solutions of liquid and acetonitrile (CH3CN) in a ratio of 1:99 and pure naphthalene crystals in a ratio of 1: 2000 in acetonitrile (CH3CN) were prepared.Quantitative analysis of indene and naphthalene in the chemical analysis department of the laboratory “Experimental Biology” on the device Prominence LC-2030C 3D Plus (Shimadzu, Japan) and analytical column (250 mm x 4.6 mm, 5 μm Brownlee ™ HPLC column, Perkin Elmer). A 10 μL / min sample solution was added to the column at a gradient flow of 1.0 ml / min of the excitable phase mixture of acetonitrile and water (85:15). The temperature in the column was set to 40°C. A total of 30 min is spent for each sample extract from the mobile phase with multi-stage gradient conditions.PDA diode - matrix absorption detector is used at a wavelength of 254 nm to increase sensitivity and selectivity. The second detector used is the fluorescent detector used in the RF-20Axs monochromator drive and emission wavelength program. This allows the best sensitivity to be achieved using the detector. As a result, it allows the detection of all types of PAUs, including inden and naphthalene.Standard samples of inden and naphthalene for analysis were solutions of different concentrations from Inden (193828 - 1G> 98% HPLC, Sigma Aldrich) and naphthalene (147141 - 25G> 99% HPLC, Sigma Aldrich): 0.05mg / ml 0.1mg / ml and 0.2 mg / ml solutions were prepared and calibrated. During the calibration of the standards, inden and naphthalene were found to have good absorption in the PDA diode-matrix absorption detector. Sample analysis was also performed on this detector. The samples isolated for analysis were analyzed based on the above method.
|
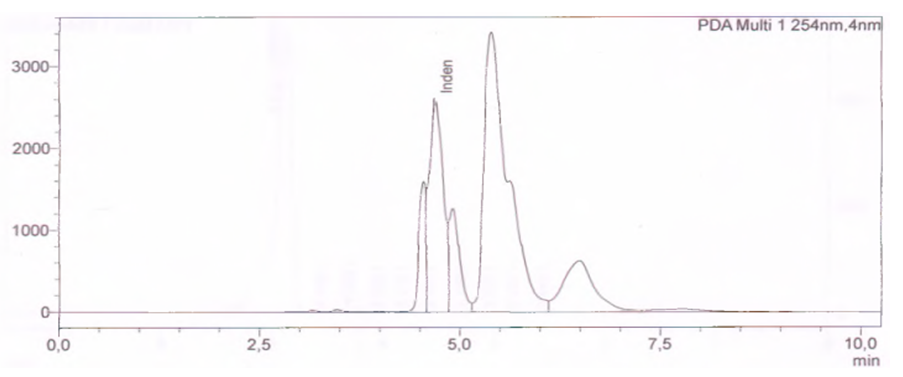 | Figure 1. Quantitative analysis of indene in the initial fraction of heavy pyrolysis oil |
|
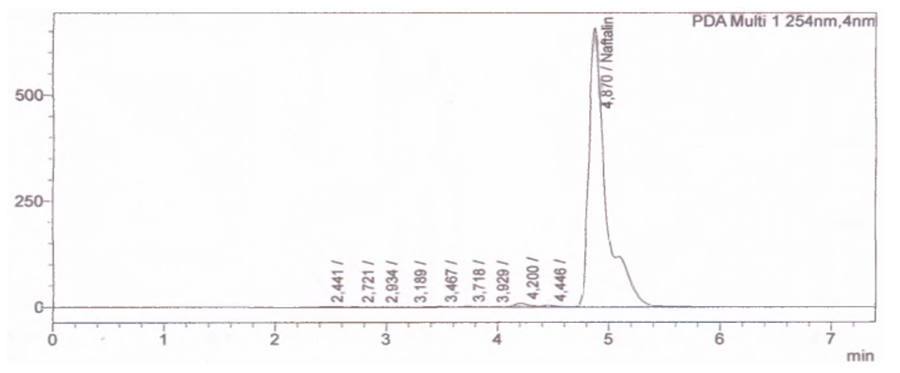 | Figure 2. Quantitative analysis of naphthalene isolated from heavy pyrolysis oil |
|
3. Conclusions
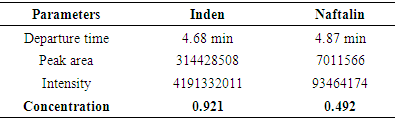
- The aim of this work is based on the quantitative analysis of polycyclic compounds, including indene and naphthalene, separated by thermal fractionation from heavy pyrolysis oil by the HELC method in the Prominence-i LC-2030C 3D Plus (Shimadzu) device. Based on the results obtained, polycyclic compounds present in heavy pyrolysis oil: naphthalene at 4.68 min, PDA diode-matrix absorption UV detectors at 4.87 min are mainly sensitive to wavelength PAHs at 254 nm detected in the detector. The results of the study showed the potential of industrial processing and creation of raw material base from polycyclic compounds in pyrolysis oil by determining the quantitative parameters of indene and naphthalene.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML