-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
International Journal of Materials and Chemistry
p-ISSN: 2166-5346 e-ISSN: 2166-5354
2022; 12(1): 1-9
doi:10.5923/j.ijmc.20221201.01
Received: Dec. 13, 2021; Accepted: Dec. 27, 2021; Published: Jan. 13, 2022

Super Absorbent Hydrogel Derived from Activated Charcoal Functionalized with Ethylenediamine and Cross-linked with Maleic Acid
Titus M. Kasimu1, Harun M. Mbuvi1, Francis M. Maingi2
1Department of Chemistry, Kenyatta University Nairobi, Kenya
2Department of Science Technology and Engineering, Kibabii University Bungoma, Kenya
Correspondence to: Francis M. Maingi, Department of Science Technology and Engineering, Kibabii University Bungoma, Kenya.
| Email: |  |
Copyright © 2022 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Superabsorbent hydrogels represent a set of polymeric materials with three-dimensional networks capable of holding a huge amount of water due to their hydrophilic nature in their structure. Their application in industries and the environment is of prime importance. This study reports the synthesis and characterization of superabsorbent hydrogels derived from activated charcoal. The activated charcoal (AC) was functionalized with ethylenediamine (EA) using sodium hydroxide as a catalyst in the absence and presence of maleic acid as a cross linker to synthesize HCE-1 and HCE-2 superabsorbent hydrogel respectively. Characterization of the hydrogels was done using Fourier transform infrared (FT-IR) spectroscopy, scanning electron microscope (SEM), and X-ray diffraction (XRD). The synthesis conditions producing optimal swelling capacity were studied by varying contact time and dosage of both activated carbon and the maleic acid. The results showed presence of C-N- stretching vibration at 1590.99 cm-1 in FT-IR spectrum of HCE-1 indicating interlinking between AC and EA monomers. XRD analysis showed a shift from amorphous to crystalline upon crosslinking. SEM analysis showed dense mast homogenous morphology with clear pores network in HCE-2 compared to the rigid rough surface observed in HCE-1 hydrogel. The dose ratio of AC: EA: maleic acid of 6:5:2 produced hydrogel with highest water absorption capacity of 1089.7±0.6%. Crosslinking the hydrogel with maleic acid was found to improve the water absorption capacity of the absorbent. The study provides a baseline for the application of the hydrogel in agriculture, especially in semi and arid regions.
Keywords: Activated charcoal, Characterization, Crosslinking, Ethylenediamine, Superabsorbent hydrogel
Cite this paper: Titus M. Kasimu, Harun M. Mbuvi, Francis M. Maingi, Super Absorbent Hydrogel Derived from Activated Charcoal Functionalized with Ethylenediamine and Cross-linked with Maleic Acid, International Journal of Materials and Chemistry, Vol. 12 No. 1, 2022, pp. 1-9. doi: 10.5923/j.ijmc.20221201.01.
Article Outline
1. Introduction
- Absorbent hydrogels are 3D lattice polymers with cross-linked hydrophilic functional groups and are insoluble in water [1]. They can absorb and retain water at least 400 times its original weight. They also make at least 95 percent of their stored water available for absorption [2]. Hydrogels are usually prepared from polar materials and are classified as physically and chemically cross-linked gels. Physically, cross-linked gels have their networks held together by physical forces, including ionic, H- bonding, or hydrophobic forces, while chemically cross-linked gels have covalently cross-linked networks [3]. Most of the hydrogels reported in the literature are synthetic non-biodegradable organic compounds [4]. This becomes the driving force for researchers to develop superabsorbent hydrogels from locally available natural materials which are degradable especially starch and chitosan among others [5]. Hydrogels have varied applications. Most common are; hygiene napkins, disposal diapers, soil for horticulture and agriculture, water and food purification [6]. Currently, hydrogels are applied mostly in technological fields including medicine [3], agriculture and horticulture [7], food packaging [8], and wastewater treatment [5]. These materials can absorb water 20 times more than their weights and are therefore referred to as super-absorbent [9]. Low-cost hydrogels with high swelling capacities have attracted more emphasis in the field of agriculture [10]. This study was geared towards the synthesis of super hydrogel absorbers from biodegradable materials for possible application in agriculture. The main chain polymer was obtained through interlinking carboxylate functional groups in AC and amine groups in EA. The maleic acid was used as a binding molecule between the polymeric units formed through ester cross-linkage. The hydrogel formed will have an increased number of hydrophilic groups and hence resulting in increased water absorption capacity.
2. Materials and Methods
2.1. Reagents and Chemicals
- The Coconut shells were obtained from Mombasa County-Kenya and transported to Kenyatta University laboratory. Ethylenediamine used was obtained from Merck Chemical Co (Darmstadt, Germany), potassium manganate VII, sodium hydroxide, and sulphuric acid were obtained from Kenya science chemical limited Kenya, while maleic acid was obtained from Sigma Aldrich Company (Germany).
2.2. Preparation of Activated Carbon
- The preparation procedure of activated charcoal was adopted from Papita [11]. Pieces of dry coconut shells were placed into a 20-L aluminum container and excess air was removed by warming. The container was then tightly closed and heated at a temperature of 473 to 553K for 8 hours after which cooling was done for 24 hours to obtain carbonized charcoal [11]. The charcoal was crushed and grounded into powder using a Sheller machine (Honda ESB 501). 20 g of the powdered charcoal was then put in a 1 L Erlenmeyer flask and 500 mL of 2M solution of acidified potassium permanganate was added. The mixture was allowed to stand for 12 hours to oxidize carbon. Oxidative reaction introduces oxygen functional groups on the surface of carbon materials to enhance functionalizing ability with ethylenediamine [9]. The mixture was then filtered and the residue was washed with deionized water to remove the purple color of the oxidizing agent and air-dried in the open to a constant weight to obtain activated carbon.
2.3. Functionalization of Activated Carbon with Ethylenediamine to Form Hydrogel HCE-1
- The procedure for the functionalization of activated charcoal with ethylenediamine was adopted from Basri et al. [12] with slight modification. The process occurred through interlinking the carboxylic acid group in AC with the amine group in EA to form a monomeric unit through an amide linkage. Accurately weighed 80.0 g of powdered AC using a weighing balance with precision (CZ 200) was transferred to a 1000 mL Erlenmeyer flask. 150 mL of deionized water was then added while stirring to form a carbon suspension. The obtained mixture was heated to a temperature of 100°C for 5 minutes followed by the addition of 50.0 g of ethylenediamine and 2.0 g of sodium hydroxide pellets as the activator. The heating of the resultant mixture at a temperature of 150°C was done until a paste formed, after which it was allowed to cool to room temperature to form a solid hydrogel polymer labeled HCE-1.
2.4. Crosslinking the Hydrogel HCE-1 with Maleic Acid
- The cross-linked HCE-2 superabsorbent hydrogel was prepared by adding 10.0 g of maleic acid to boiling HCE-1. Stirring and heating of the mixture was done continuously at a temperature of 150°C until viscous gel was formed. The gel was then cooled at room temperature to form superabsorbent hydrogel labeled HCE-2. Ester linkage was facilitated by the availability of hydroxyl groups in the polymeric material HCE-1 and carboxylate groups in maleic acid. Figure 1 shows the diagrammatic scheme for the preparation of the hydrogel.
2.5. Characterization of the Hydrogel
2.5.1. Fourier Transform Infrared (FT-IR) Analysis of the Superabsorbent Hydrogels
- The procedure was adopted from Demitri et al. [13] to determine functional groups present in the hydrogels absorbers. Crystal of the superabsorbent hydrogels with and without cross-linker HCE-2 and HCE-1 respectively were dried in the open air for 12 hours until they attained constant weight. 1 mg of each of the hydrogel was mixed with 25 mg of dry spectroscopic grade potassium bromide. The mixtures were ground using pestle and mortar to form a fine powder. The powder was compressed into thin pellets and investigated for functional groups using FT-IR (Shimadzu IR Tracer-100) at a wavelength range of 4000-200cm-1 [14].
2.5.2. Phase Composition Analysis of the Superabsorbent Hydrogels
- The procedure for phase analysis of the hydrogels HCE-1 and HCE-2 was adopted from Wanrosli et al. [15]. Analysis was done using XRD (Hossein Beygin) at scattering angle (2θ) ranging from 10 to 90° and at a scan rate of 5°/min.
2.5.3. Microstructural Analysis of the Superabsorbent Hydrogels
- The microstructural analysis of HCE-1 and HCE-2 hydrogel was determined by soaking them in buffer solutions of pH 11 for 12 hours. The mixture was then filtered using filter paper no 41 and residue dried in the oven at 45°C until a constant weight was obtained. Dried hydrogels were coated with gold. The gold-plated stubs were then placed in a SEM chamber (Zeiss Supra 60) at an accelerating voltage of 250 kV to obtain SEM micrographs [16].
2.6. Effect of Mass of AC (g) on the Swelling Capacity of Cross-linked Hydrogel
- The hydrogels were prepared by varying mass of AC from (1, 2, 4, 6, 8, and 10 g) while maintaining the mass of ethylenediamine at 5.0 g, maleic acid binder at 2.0 g, and 1.0 g of NaOH activator. 2.0g of each prepared sample was then immersed in water for 24 hours to determine the swelling capacity.
2.7. Effect of Cross-linker on the Swelling Capacity of the Hydrogel
- The effect of the cross-linker was studied in triplicates by varying the mass of maleic acid (0.5, 1.0, 1.5, 2.0, 2.5, and 3.0 g) while maintaining the mass of activated carbon as 6 g, the mass of ethylenediamine as 5.0 g, and the mass of sodium hydroxide activator as 1.0g. 2.0 g of each prepared sample was then immersed in water for 24 hours to determine the swelling capacity as described in equation 1 provided in section 2.9.
2.8. Effect of Swelling Time on the Swelling Capacity of the Hydrogel HCE-2
- The effect of swelling time on swelling of superabsorbent hydrogels HCE-2 was studied in triplicates by varying time (0.5, 1, 2, 4, 6, 12, and 24 hours). 2.0 g of each hydrogel prepared at the optimum ratio of AC: EA: maleic acid of 6:5:2 was immersed in 500 mL for a specified swelling time. The samples were then periodically removed after the expiry of contact time and its new mass determined. The swelling capacity was then determined using equation 1 provided in section 2.9.
2.9. Equilibrium Water Content (EWC)
- The procedure for determining equilibrium water content (EWC) was adopted from [16]. 2.0 g of the hydrogel polymers were put in polyester bags. The weight of the hydrogels and polyester bags was determined and recorded as (Ws). The polyester bags containing hydrogels were then immersed in 500 mL of distilled water and allowed to absorb water for 24 hours. After the swelling period, the samples were removed and excess water on the surface of polyester bags was removed using tissue paper and their weight determined and recorded as (Wd) [16]. Equation 1 was used to determine the equilibrium water content.
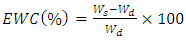 | (1) |
3. Results and Discussions
3.1. FT-IR Spectrum of Superabsorbent Hydrogels
- Analysis using FT-IR was carried out to identify the functional groups present in hydrogel super absorbers before and after cross-linking. Figure 1 shows the IR spectrum of the uncross-linked hydrogel HCE-1 and cross-linked hydrogel (HCE-2).
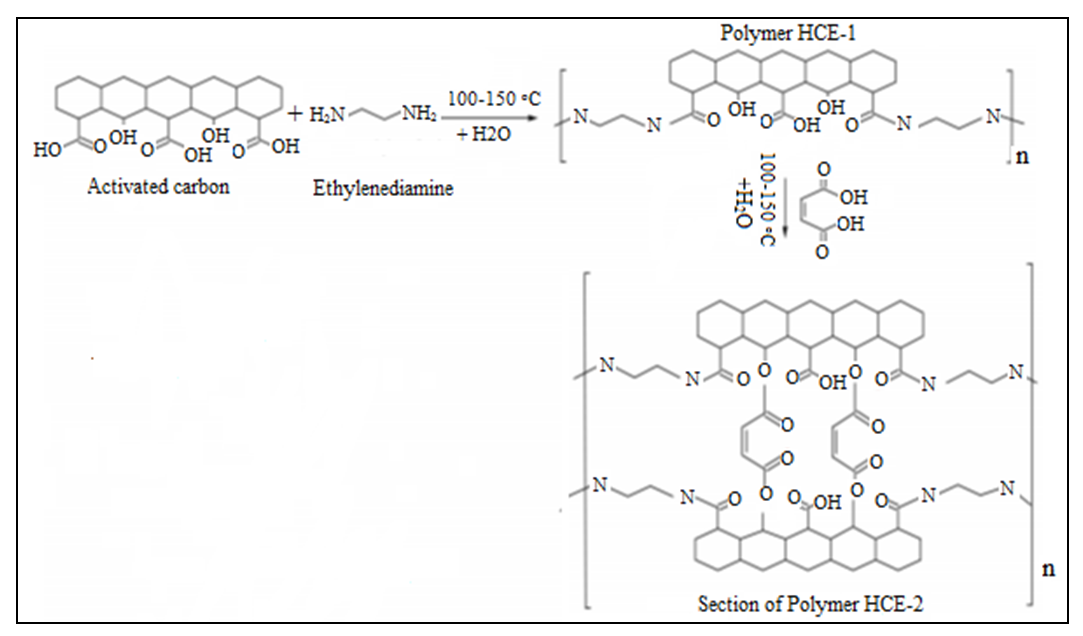 | Figure 1. Diagrammatic scheme for the preparation of hydrogel |
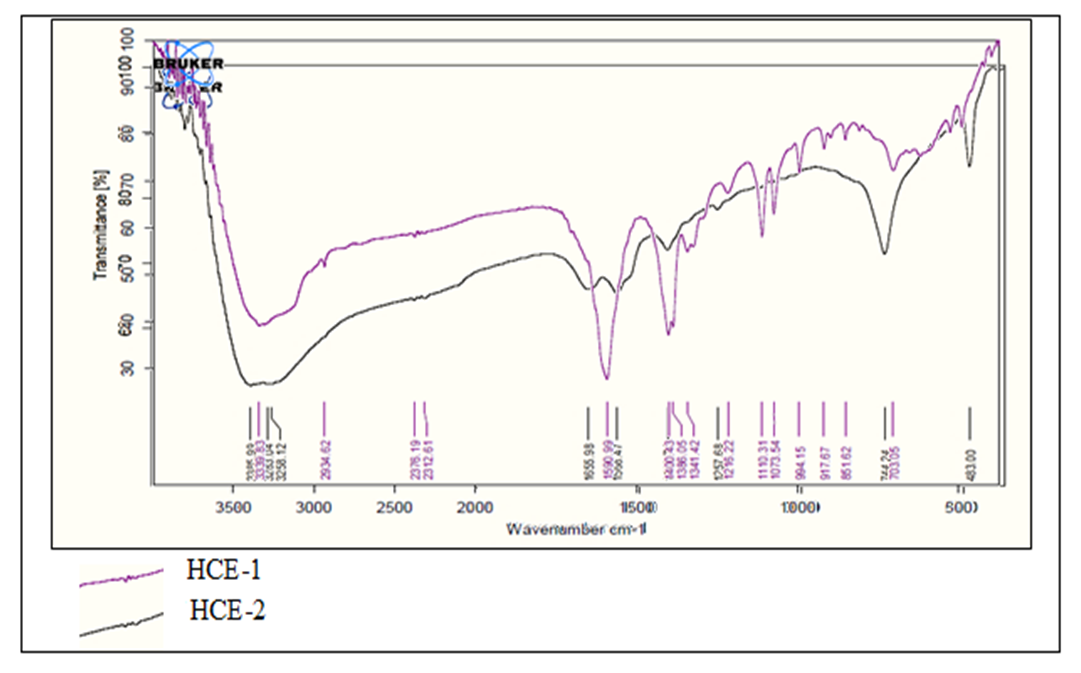 | Figure 2. FT-IR Spectrum of HCE-1 and HCE-2 hydrogels |
3.2. Phase Composition of HCE-1 and HCE-2 Hydrogels
- Figures 3 and 4 show the diffraction patterns of super hydrogel absorbers HCE-1 and HCE-2 respectively obtained during the analysis. The amorphous hump observed between angle (2θ) 10 and 31° in figure 3 and the few crystalline phases shows that HCE-1 was semi-crystalline [25].
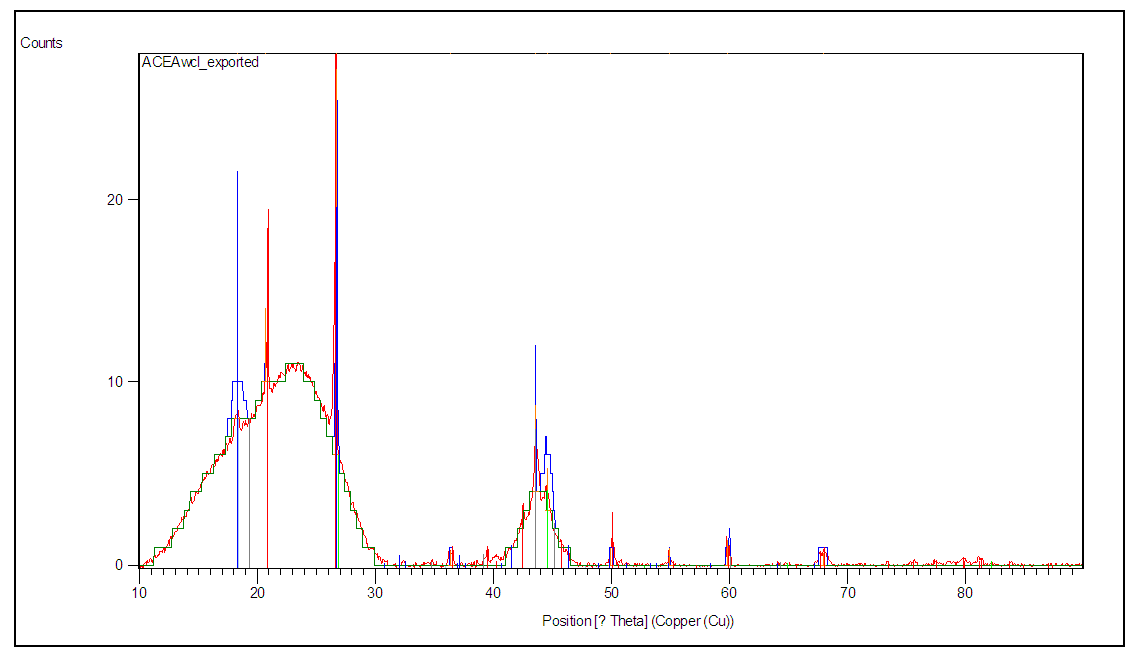 | Figure 3. Powdered diffraction pattern of HCE-1 Hydrogel |
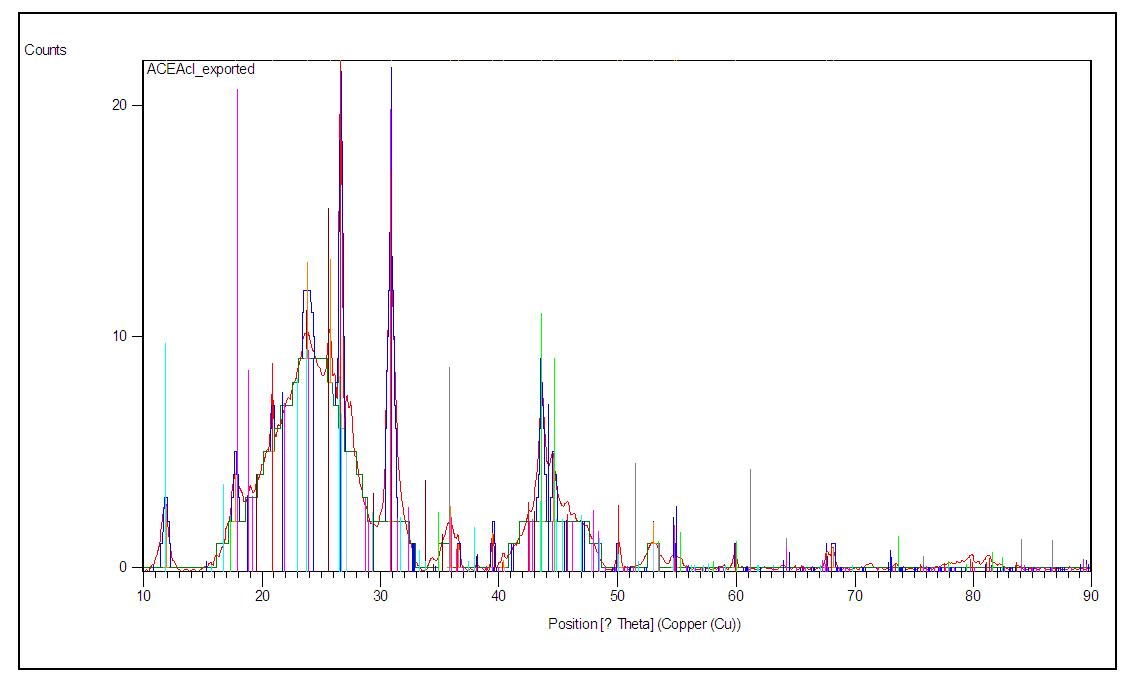 | Figure 4. Powdered diffraction pattern of HCE-2 hydrogel |
3.3. Analysis of Microstructure in the Hydrogel Using Scanning Electron Microscope (SEM)
- The microstructure and morphological analysis of the hydrogel absorbers of HCE-1 and HCE-2 was done using a scanning electron microscope (SEM) (model Zeiss supra 60) at an accelerating voltage of 2.50 kV. The SEM images of powdered hydrogels are shown in figure 5.
 | Figure 5. SEM micrographs of HCE-1 (A) and HCE-2 (B) hydrogels |
3.4. Effect of Amount of AC Used on the Swelling Capacity of Hydrogel (HCE-2)
- Figure 6 shows the mean percentage swelling obtained when 2.0 g of prepared hydrogel was immersed in distilled water as described in section 2.6.
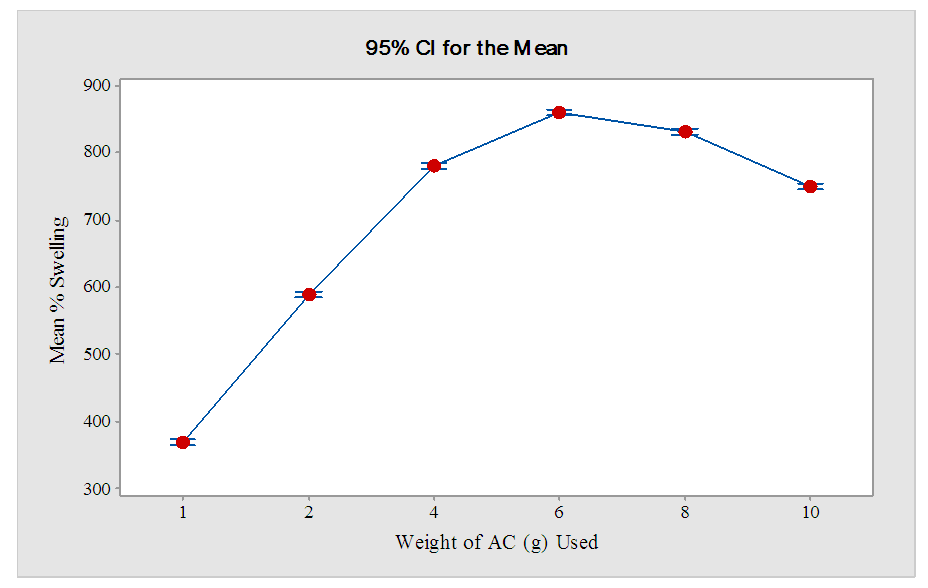 | Figure 6. Effect of amount of AC (g) on % swelling of 2 g of HCE-2 |
3.5. Effect of Amount of Maleic Acid (g) Used on the Swelling Capacity of Hydrogel (HCE-2)
- Figure 7 shows the effect of varying amount of maleic acid cross linker on the mean swelling capacity of super absorber hydrogel HCE-2.
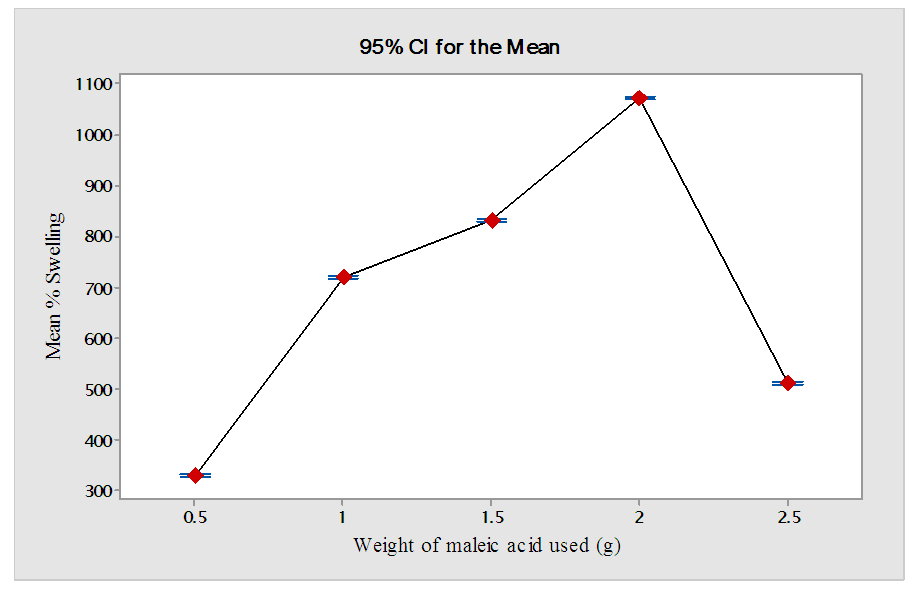 | Figure 7. Effect of weight of maleic acid (g) on % swelling of HCE-2 |
3.6. Effect of Swelling Time on the Swelling Capacity of the Hydrogel
- Figure 8 shows the effect of swelling time on the swelling capacity of super absorbent hydrogels HCE-2 at different contact times.
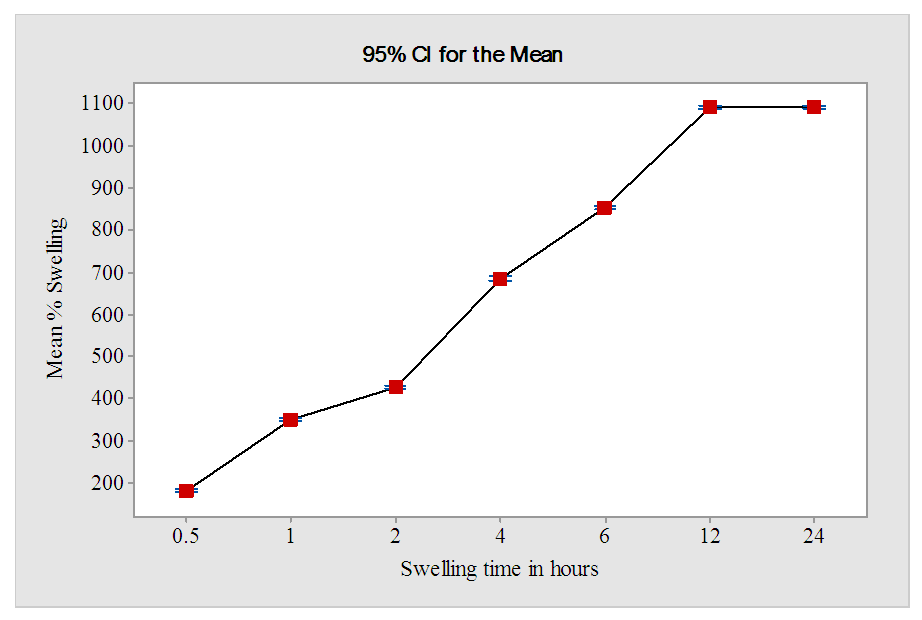 | Figure 8. Effect of swelling time on % swelling of 2 g of HCE-2 at optimum ratio of AC:EA:maleic acid of 6:5:2 |
4. Conclusions
- The results from this study showed that a mass ratio of 6:5:2 between AC with ethylenediamine and maleic acid produced hydrogel with maximum water absorption as well as swelling capacity. The FT-IR spectra sharp bands at 1216.2 cm-1 in HCE-1 a credited to N-H bending bond is proof of interlink between AC and EA monomers. Increased number of -OH functional groups coupled with a sharp peak at 1655.98 cm-1 associated with CO stretching in amide, showed successful ester crosslinking in HCE-2 hydrogel during the synthesis process. XRD analysis showed an increased crystallinity in HCE-2 polymer as compared to the amorphous structure in HCE-1 hydrogel. SEM analysis of showed crystalline intact and rigid structure in HCE-1 with fewer pores on their surface compared to the well-developed fibrous with smooth irregular pores and lamina structure in HCE-2. A maximum mean swelling capacity of 1089.7 ± 0.6% of HCE-2 when subjected to a contact time of 12 hours in 500 mL deionized water was achieved. HCE-2 being a good water absorber has greater potential in boosting agriculture, especially in arid and semi-arid regions by improving water retainability in soils during dry periods.
Declaration of Interests
- The authors declare that they have no known competing financial interests or personal relationships that could appear to have influenced the work described in this paper.
ACKNOWLEDGEMENTS
- The authors’ expresses gratitude to the department of chemistry of Kenyatta University and department of science technology and engineering of Kibabii University for assistance offered during the research period.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML