-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
International Journal of Materials and Chemistry
p-ISSN: 2166-5346 e-ISSN: 2166-5354
2021; 11(2): 17-27
doi:10.5923/j.ijmc.20211102.01
Received: Nov. 14, 2021; Accepted: Dec. 6, 2021; Published: Dec. 29, 2021

Evaluation of Corrosion Inhibition of Aluminum by 2-(4-methylbenzylthio)-3- nitroimidazo [1, 2, a]pyridine in Hydrochloric Acid Medium
Ehouman Ahissan Donatien1, Zran Vanh Eric-Simon2, Coulibali Siomenan2, Bamba Amara2, Douan Katy Mao2, Ablo Evrad2, Diabate Massogbè3, Kouakou Adjoumani Rodrigue1, Niamien Paulin Marius2
1Laboratoire de Thermodynamique et Physico-Chimie du Milieu (LTPCM), UFR SFA, Université NANGUI ABROGOUA, Abidjan, Côte-d’Ivoire
2Laboratoire de Constitution et Réaction de la Matière (LCRM), UFR SSMT, Université Félix Houphouët-Boigny, Abidjan Cocody, Abidjan, Côte-d’Ivoire
3Laboratoie de Biochimie Alimentaire et de Technologies des Produits Tropicaux (LBATPT), UFR STA, Université NANGUI ABROGOUA, Abidjan, Côte-d’Ivoire
Correspondence to: Ehouman Ahissan Donatien, Laboratoire de Thermodynamique et Physico-Chimie du Milieu (LTPCM), UFR SFA, Université NANGUI ABROGOUA, Abidjan, Côte-d’Ivoire.
| Email: |  |
Copyright © 2021 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

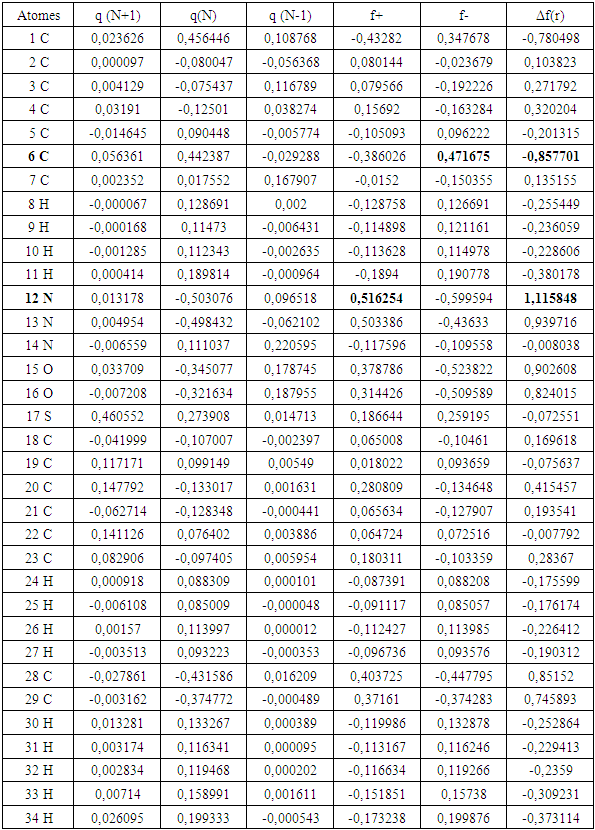
Due to its extensive use, the behaviour of aluminium in 1M hydrochloric acid solution was studied in depth in this work. This study which is mainly based on the inhibition properties of 2-(4-methylbenzylthio)-3- nitroimidazo [1, 2, a]pyridine (MTNI) was carried out using the mass loss technique and the theoretical method based on density functional theory (DFT) at the B3LYP level with the base 6-311G (d, p). The inhibitory efficiency of the molecule increases with increasing concentration and decreases with increasing temperature. Adsorption isotherm studies revealed that the molecule adsorbs on the aluminium surface according to the modified Langmuir isotherm or the Villamil isotherm. The Adejo-Ekwenchi isotherm indicates that the adsorption of MTNI is dominated by physisorption. The thermodynamic quantities of adsorption and activation were determined and discussed. The calculated quantum chemical parameters related to the efficiency of inhibition are the energy of the highest occupied molecular orbital E(HOMO), the energy of the lowest unoccupied molecular orbital E(LUMO), the HOMO-LUMO energy gap, the hardness (η), softness (S), dipole moment (μ), electron affinity (A), ionization energy (I), absolute electronegativity (χ), absolute electronegativity (χ), fraction (ΔN) of electrons transferred from MTNI to aluminium and electrophilicity index(ω). The local reactivity was analyzed through the condensed Fukui function and condensed softness indices to determine the nucleophilic and electrophilic attack sites. The theoretical results are consistent with the reported experimental data.
Keywords: Corrosion inhibition, Aluminium, Density functional theory (DFT), Mass loss method, 2-(4-methylbenzylthio)-3- nitroimidazo [1, 2, a]pyridine
Cite this paper: Ehouman Ahissan Donatien, Zran Vanh Eric-Simon, Coulibali Siomenan, Bamba Amara, Douan Katy Mao, Ablo Evrad, Diabate Massogbè, Kouakou Adjoumani Rodrigue, Niamien Paulin Marius, Evaluation of Corrosion Inhibition of Aluminum by 2-(4-methylbenzylthio)-3- nitroimidazo [1, 2, a]pyridine in Hydrochloric Acid Medium, International Journal of Materials and Chemistry, Vol. 11 No. 2, 2021, pp. 17-27. doi: 10.5923/j.ijmc.20211102.01.
Article Outline
1. Introduction
- Pure aluminium is a remarkable metal because it is resistant to corrosion. It is currently the most widely used non-ferrous metal [1] because of its low density and its electrical and thermal performance. It is most often used in industries related to transportation: aviation, automotive, packaging, construction, mechanical, etc. However, when it comes into contact with aggressive media (acids, bases, etc.), its thin protective layer (a few micrometers thick) is destroyed and the material undergoes an alteration (corrosion) which leads to the loss of its physical and chemical properties. Corrosion results from the chemical or electrochemical action of an environment on metals and alloys. The consequences are important in various fields and in particular in industry: production stoppages, replacement of corroded parts, accidents and pollution risks are frequent situations with sometimes heavy economic consequences [2]. The most effective way of combating this phenomenon is undoubtedly the use of organic molecules (corrosion inhibitors) acting at the metal-environment interface. Several organic molecules have been tested as inhibitors of aluminium corrosion in acidic environments. Thus, hydrazines [3], Schiff bases [4,5], therapeutic molecules [6-9], plant extracts [10-12], etc., have been used successfully.This work, which is a contribution to the study of corrosion inhibition of metals in acidic media, aims to study the behaviour of 2-(4-methylbenzylthio)-3-nitroimidazo [1, 2, a] pyridine (MTNI) towards the corrosion of aluminium in 1M hydrochloric acid.
2. Experimental Details
2.1. Materials and Sample Preparation
- The molecule used as an inhibitor in this work, namely 2-(4-methylbenzylthio)-3-nitroimidazo [1, 2, a] pyridine (MTNI) with chemical formula C15H13N3O2S and molar mass M = 299.35g/mol, was synthesized at the former Laboratory of Structural Organic Chemistry (LCOS) of the University Félix Houphouët Boigny of Abidjan-Cocody and its molecular structure was identified by 1H, 13C RMN spectroscopy. It is in the form of a whitish powder. The molecular structure is shown in Figure 1.
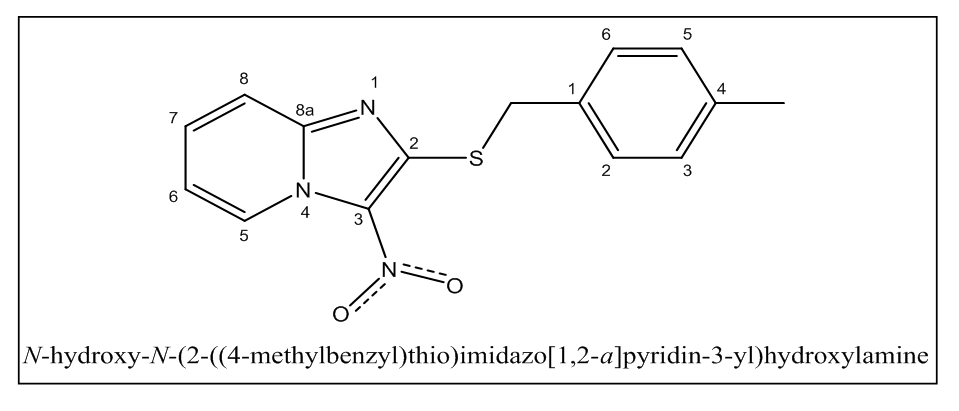 | Figure 1. Molecular structure of 2-(4-methylbenzyl) thio-3-nitroimidazo [1,2-a]pyridine |
2.2. Mass Loss Method
- Mass loss measurements were carried out by total immersion of the pre-weighed aluminium sample in 100 mL capacity beakers containing 50 mL of the test solution maintained at a temperature of (298K to 323K). The samples were recovered one hour later and rinsed thoroughly with distilled water, cleaned, dried in acetone and reweighed using a balance with a sensitivity of ±0.1 mg. All tests were performed in triplicate to ensure reliability of the results. Weight loss was considered as the difference between the initial weight and the weight after 1 h of immersion. The average values of the mass loss data were used to calculate parameters such as corrosion rate, inhibition efficiency and surface coverage using the following relationships:
 | (1) |
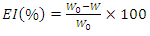 | (2) |
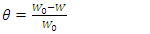 | (3) |
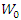 and
and  are the corrosion rate in the absence and presence of the inhibitor respectively∆m is the mass loss, S is the total surface area of the copper sample and t is the immersion time.
are the corrosion rate in the absence and presence of the inhibitor respectively∆m is the mass loss, S is the total surface area of the copper sample and t is the immersion time.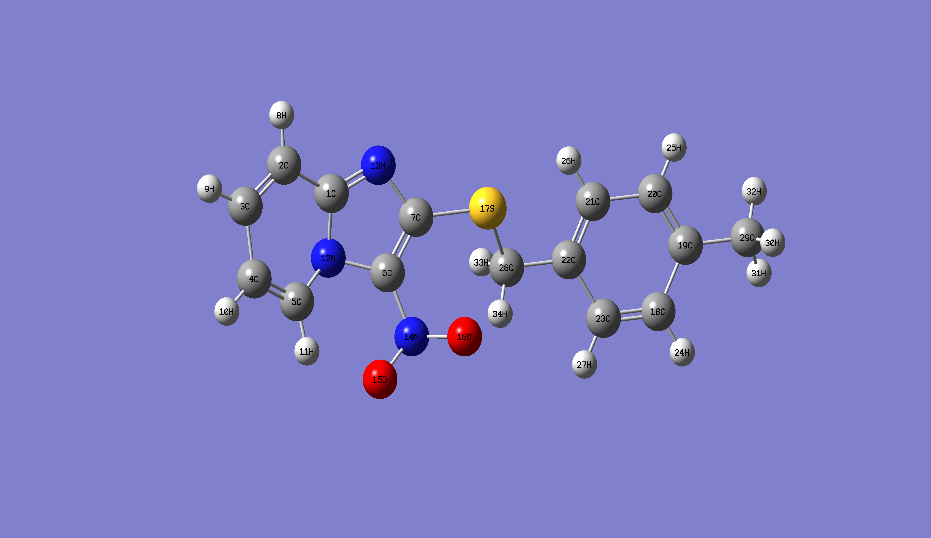 | Figure 2. Optimized structure of 2-(4-methylbenzylthio)-3-nitroimidazo [1, 2, a]pyridine calculated by B3LYP/6-311G (d, p) |
2.3. Quantum Chemistry Calculations
- In order to explain the most important electronic effects exhibited by 2-(4-methylbenzylthio)-3-nitroimidazo [1, 2, a] pyridine (MTNI) in the inhibition of aluminium corrosion, we calculated the quantum chemical parameters. All calculations were performed in the gas phase using the Gaussian 09 software [13]. By improving the calculation method, density function theory (DFT) was widely used due to its accuracy and low computational cost to calculate a wide variety of molecular properties and provided reliable results that are consistent with the experimental data [14]. The molecular configuration of the inhibitor was geometrically optimized by this theory (DFT) with the B3LYP functional [15] (three Becke parameters with hybrid Lee-Yang-Parr correlation function) on a 6-311 G (d, p) basis set.The basic relationship of the density functional theory of chemical reactivity is precisely, that established by Parr et al [16] which links the chemical electron potential
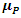 to the first derivative of the energy with respect to the number of electrons
to the first derivative of the energy with respect to the number of electrons  , and thus with the negative of the electronegativity
, and thus with the negative of the electronegativity  :
: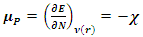 | (4) |
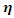 which measures both the stability and reactivity of a molecule [17] has been defined as the second derivative of the total energy
which measures both the stability and reactivity of a molecule [17] has been defined as the second derivative of the total energy 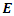 with respect to
with respect to 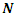 at constant external potential
at constant external potential 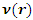 :
: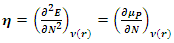 | (5) |
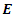 is the total energy,
is the total energy, 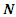 is the number of electrons and
is the number of electrons and 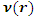 is the external potential.According to Koopmans theorem [18], the ionization energy I can be approximated as the negative of the energy of the highest occupied molecular orbital (HOMO):
is the external potential.According to Koopmans theorem [18], the ionization energy I can be approximated as the negative of the energy of the highest occupied molecular orbital (HOMO):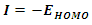 | (6) |
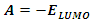 | (7) |
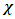 and the hardness
and the hardness 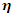 can then be written as follows:
can then be written as follows: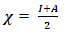 | (8) |
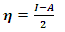 | (9) |
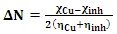 | (10) |
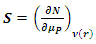 | (11) |
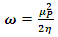 | (12) |
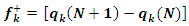 | (13) |
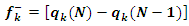 | (14) |
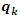 is the gross charge of atom
is the gross charge of atom 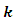 in the molecule and
in the molecule and 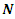 is the number of electrons.
is the number of electrons.3. Results and Discussion
3.1. Mass Loss Experiment
- Mass loss data were determined at the end of a 1 hour time interval in the absence and presence of different concentrations of 2-(4-methylbenzylthio)-3-nitroimidazo [1, 2, a]pyridine (MTNI) and were used to calculate corrosion rates, inhibition efficiency and degree of surface coverage, according to equations (1 to 3). Figure 3 shows the evolution of the corrosion rate with concentration and temperature respectively. Examination of Figure 3 shows that the corrosion rate increases with temperature for all concentrations and decreases with increasing inhibitor concentration. In the absence of the inhibitor, the corrosion rate is very high, which shows that the addition of MTNI to the corrosive medium delays the corrosion of aluminium. In addition, the presence of MTNI promotes the formation of a protective layer due to the adsorption of the molecule on the metal surface. This protective layer prevents the aluminium from losing sufficient electrons or undergoing strong dissolution in acid. These results indicate that the molecule studied has a good corrosion inhibition performance of aluminium in hydrochloric acid solution.
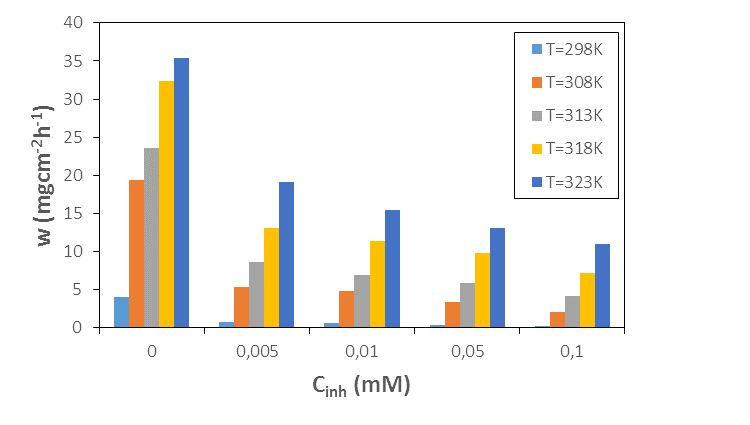 | Figure 3. Corrosion rate versus MTNI concentration curve at different temperatures |
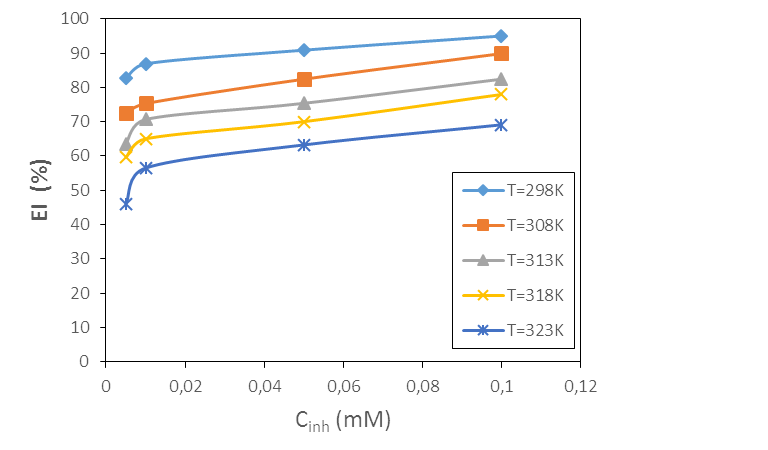 | Figure 4. Inhibition efficiency versus MTNI concentration at different temperatures |
3.2. Study of Adsorption Isotherms and Thermodynamic Adsorption Quantities
- The study of adsorption isotherms involved in the process of inhibiting metal corrosion by organic molecules shows how these compounds attach to the surface of a metal. Indeed, the adsorption of an organic adsorbate on the metal-solution interface can be likened to a chemical reaction in which water molecules adsorbed on the metal surface are replaced by the organic molecules from the solution. It is therefore a phenomenon of substitutional adsorption [22,23] as shown by the following reaction equation:
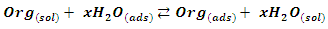 | (4) |
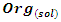 and
and 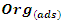 are respectively the organic molecule in solution and the organic molecule adsorbed on the metal surface,
are respectively the organic molecule in solution and the organic molecule adsorbed on the metal surface, 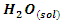 and
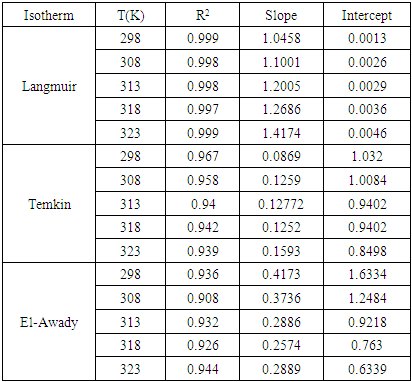
and 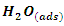 are respectively the water molecule in solution and the water molecule adsorbed, x is the number of water molecules replaced by an organic molecule.In this work, we tried various adsorption isotherms and selected those that best reflect the behaviour of MTNI on the aluminium surface. We have therefore chosen the Langmuir, Temkin and El-awady isotherms. The equations that defining these isotherms are given in Table 1.
are respectively the water molecule in solution and the water molecule adsorbed, x is the number of water molecules replaced by an organic molecule.In this work, we tried various adsorption isotherms and selected those that best reflect the behaviour of MTNI on the aluminium surface. We have therefore chosen the Langmuir, Temkin and El-awady isotherms. The equations that defining these isotherms are given in Table 1.
|
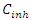 is the inhibitor concentration;
is the inhibitor concentration;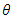 is the metal surface coverage rate;
is the metal surface coverage rate;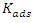 is the equilibrium constant of the adsorption process;
is the equilibrium constant of the adsorption process;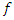 is an energy inhomogeneity factor of the surface;
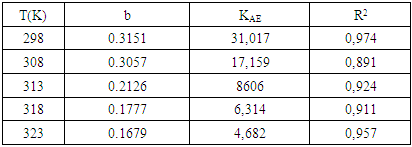
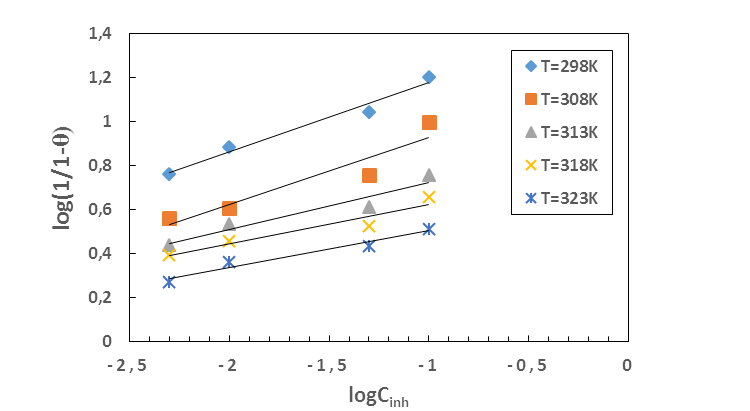
is an energy inhomogeneity factor of the surface;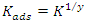 ; 1⁄y is the number of active sites occupied by an inhibitor molecule. Figures 5, 6, 7 show the representation of these different isotherms.
; 1⁄y is the number of active sites occupied by an inhibitor molecule. Figures 5, 6, 7 show the representation of these different isotherms.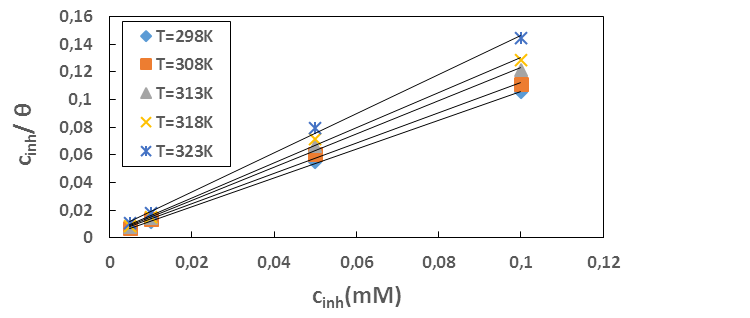 | Figure 5. Langmuir adsorption isotherm plots of MTNI on aluminium in 1M HCl |
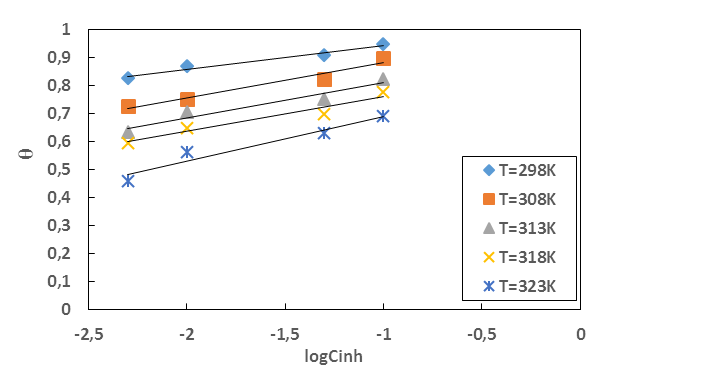 | Figure 6. Temkin adsorption isotherm plots of MTNI on aluminium in 1M HCl |
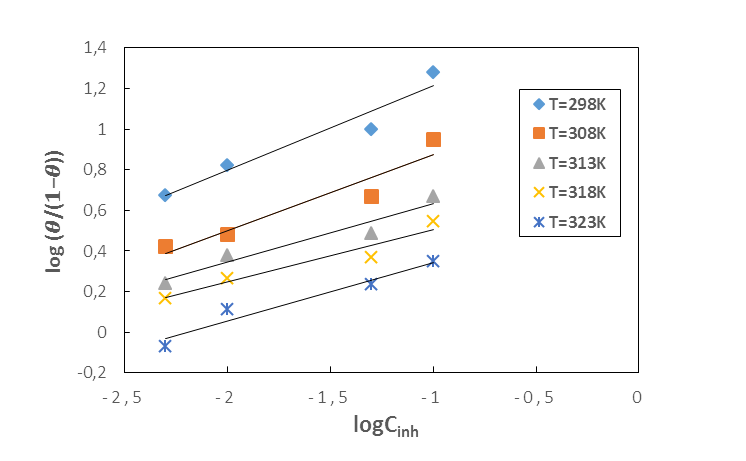 | Figure 7. El-awady adsorption isotherm plots of MTNI on aluminium in 1M HCl |
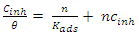 | (15) |
|
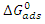 is calculated using the following relationship [28]:
is calculated using the following relationship [28]: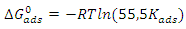 | (16) |
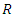 is the perfect gas constant, T is the absolute temperature and 55.5 is the concentration of water in mol.L-1 and
is the perfect gas constant, T is the absolute temperature and 55.5 is the concentration of water in mol.L-1 and 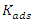 is the adsorption equilibrium constant. The values of the adsorption equilibrium constant are deduced from the parameters of the modified Langmuir isotherm (straight line intercept). The other thermodynamic parameters of adsorption (adsorption enthalpy
is the adsorption equilibrium constant. The values of the adsorption equilibrium constant are deduced from the parameters of the modified Langmuir isotherm (straight line intercept). The other thermodynamic parameters of adsorption (adsorption enthalpy 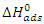 and adsorption entropy
and adsorption entropy 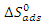 ) are calculated using the following relationship:
) are calculated using the following relationship: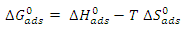 | (17) |
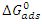 as a function of temperature (Figure 8) leads to values of
as a function of temperature (Figure 8) leads to values of 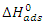 (intercept of the straight lines) and
(intercept of the straight lines) and 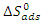 ), (the slope of the straight line). The different thermodynamic adsorption parameters are recorded in Table 3.
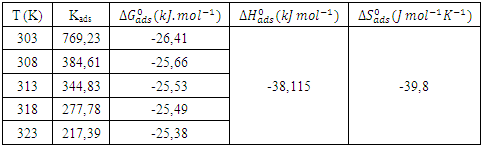
), (the slope of the straight line). The different thermodynamic adsorption parameters are recorded in Table 3.
|
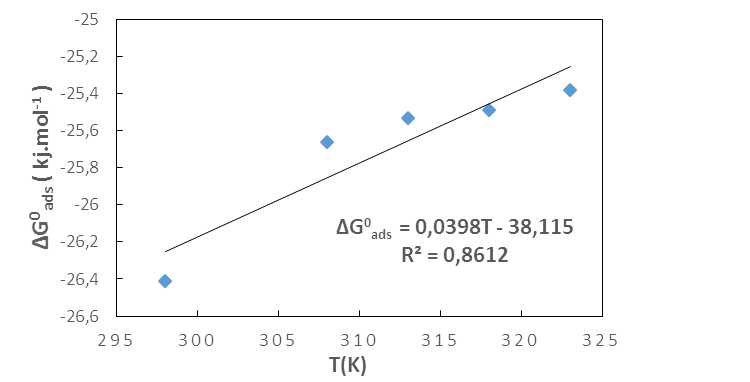 | Figure 8. Variation of  versus temperature versus temperature |
 indicate the stability of the adsorbed layer on the aluminium surface and the spontaneity of the adsorption process [29]. According to the literature [30], a value of
indicate the stability of the adsorbed layer on the aluminium surface and the spontaneity of the adsorption process [29]. According to the literature [30], a value of 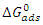 lower than -40 kJ.mol-1 would indicate a chemical adsorption process (chemisorption) whereas a value higher than -20 kJ.mol-1 would indicate a physical adsorption process (physisorption). For values between -40 kJ.mol-1 and -20 kJ.mol-1, both types of adsorption would exist. In view of the values contained in Table 3, we can deduce that the adsorption of MTNI on aluminium takes place according to the two adsorption modes (physisorption and chemisorption). The negative sign of
lower than -40 kJ.mol-1 would indicate a chemical adsorption process (chemisorption) whereas a value higher than -20 kJ.mol-1 would indicate a physical adsorption process (physisorption). For values between -40 kJ.mol-1 and -20 kJ.mol-1, both types of adsorption would exist. In view of the values contained in Table 3, we can deduce that the adsorption of MTNI on aluminium takes place according to the two adsorption modes (physisorption and chemisorption). The negative sign of 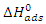 reflects the exothermic character of the adsorption of the molecule on aluminium. If the value of
reflects the exothermic character of the adsorption of the molecule on aluminium. If the value of 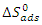 is negative, this reflects a decrease in disorder during the adsorption of the inhibitor. An increase in disorder [31] would be related to the desorption of water molecules, whereas a decrease could be related to the weakening of the adsorption force. In order to correctly justify the adsorption mode of the studied molecule, we used the Adejo-Ekwenchi isotherm [32]. Indeed, this isotherm allows us to know the adsorption mode of an organic compound. This model is based on the following equation:
is negative, this reflects a decrease in disorder during the adsorption of the inhibitor. An increase in disorder [31] would be related to the desorption of water molecules, whereas a decrease could be related to the weakening of the adsorption force. In order to correctly justify the adsorption mode of the studied molecule, we used the Adejo-Ekwenchi isotherm [32]. Indeed, this isotherm allows us to know the adsorption mode of an organic compound. This model is based on the following equation: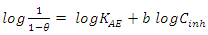 | (18) |
 | Figure 9. Adejo-Ekwenchi isotherm plots of MTNI on aluminium in 1M HCl |
|
3.3. Effect of Temperature and Activation Parameters on the Corrosion Process
- The effect of temperature on corrosion and its inhibition process for aluminium in 1M in the absence and presence of different concentrations of MTNI at different temperatures ranging from 298K to 323K was evaluated.The temperature dependence of the corrosion rate can be considered as an Arrhenius-type process, whose rate is given by [34]
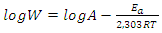 | (19) |
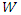 is the corrosion rate, R is the perfect gas constant, A is the frequency factor.The curve of
is the corrosion rate, R is the perfect gas constant, A is the frequency factor.The curve of 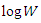 versus 1/T is given in Figure 10.
versus 1/T is given in Figure 10.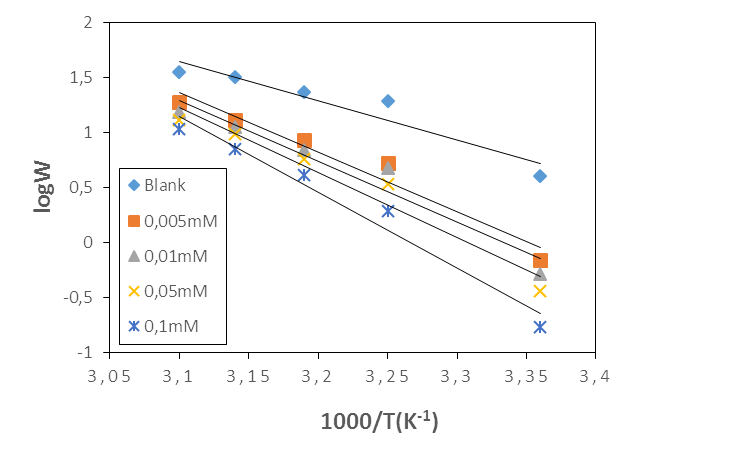 | Figure 10. Arrhenius plots for aluminium in 1M HCl in the absence and presence of MTNI |
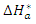 and the change in activation entropy
and the change in activation entropy 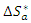 using the equation of transition states:
using the equation of transition states: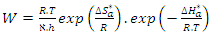 | (20) |
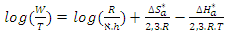 | (21) |
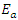 activation energy,
activation energy, 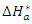 activation enthalpy variation and
activation enthalpy variation and 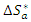 activation entropy variation. The representation of
activation entropy variation. The representation of 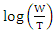 as a function of 1/T is given in Figure 11
as a function of 1/T is given in Figure 11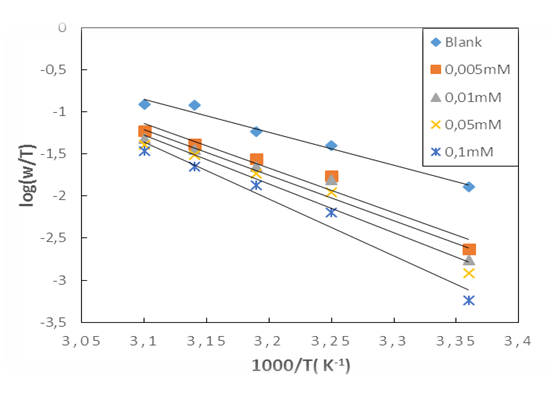 | Figure 11. Arrhenius plots for aluminium in 1M HCl in the absence and with different concentrations of MTNI |
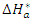 and
and 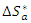 are calculated from the slope (
are calculated from the slope (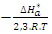 ) and intercept
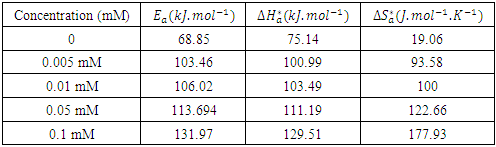
) and intercept 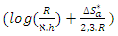 of the straight lines obtained respectively. The values obtained are recorded in Table 5.
of the straight lines obtained respectively. The values obtained are recorded in Table 5.
|
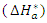 reflect the endothermic nature of the aluminium dissolution process. The values of the activation entropy variations
reflect the endothermic nature of the aluminium dissolution process. The values of the activation entropy variations 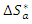 are all positive, reflecting an increase in disorder during the dissolution of aluminium which could be the reason for the decrease in inhibitory efficiency when the temperature increases.
are all positive, reflecting an increase in disorder during the dissolution of aluminium which could be the reason for the decrease in inhibitory efficiency when the temperature increases.3.4. Molecular Descriptor Parameters
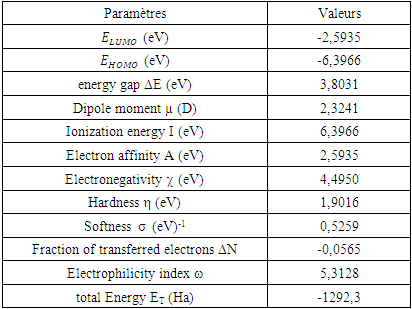
- In this study the quantum parameters were calculated using the descriptors of the conceptual density functional theory (DFT) which are very important to explain the reactivity of the molecule. In general, this theory allows to confirm the experimental results. The values of the different descriptor parameters of the molecule are reported in Table 6.
|
|
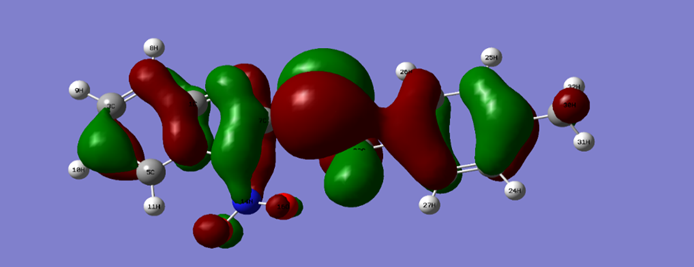 | Figure 12. HOMO density of the inhibitor at B3LYP/6-311G (d, p) |
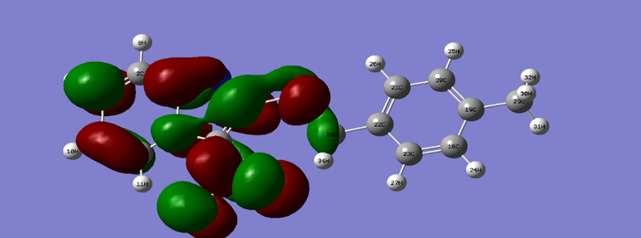 | Figure 13. LOMO density of the inhibitor at B3LYP/6-311G (d, p) |
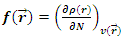 | (22) |
3.5. Mechanism of Corrosion Inhibition of Aluminum in HCl Medium by 2-(4-methylbenzylthio)-3- nitroimidazo [1, 2, a]pyridine (MTNI)
- The experimental and theoretical results show that:MTNI cannot supply electrons to aluminum (∆N<0)MTNI can receive electrons from aluminium (ω=5.3128 eV)In the presence of the hydronium ion
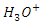
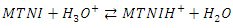
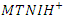 reacts with Cl- ions attached to Al+3 ionsOn some sites, the aluminium gives up electrons to the molecule (on the electrophilic part of the molecule N(13).Scheme of the mechanism
reacts with Cl- ions attached to Al+3 ionsOn some sites, the aluminium gives up electrons to the molecule (on the electrophilic part of the molecule N(13).Scheme of the mechanism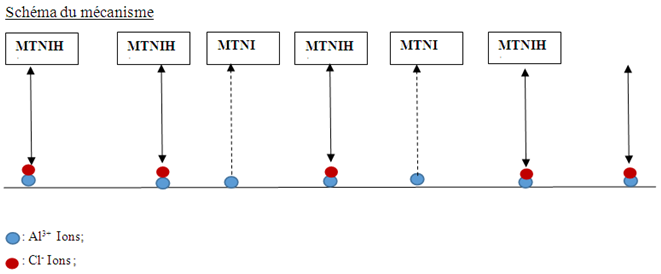 The double arrows indicate electrostatic interactions (physisorption) and the arrows with interrupted supports indicate the contribution of electrons to the molecule and formation of the TTNIAl complex.
The double arrows indicate electrostatic interactions (physisorption) and the arrows with interrupted supports indicate the contribution of electrons to the molecule and formation of the TTNIAl complex.4. Conclusions
- The mass loss technique and the DFT method were used to evaluate the corrosion inhibition of aluminium by 2-(4-methylbenzyl) thio)-3-nitroimidazo [1,2,a] pyridine (MTNI) in 1M HCl. The following conclusions can be drawn from this study:MTNI acts as a good corrosion inhibitor for aluminium in 1M hydrochloric acid.The inhibition efficiency is concentration and temperature dependent.MTNI adsorbs to aluminium according to the modified Langmuir isotherm.The calculated thermodynamic parameters related to adsorption and activation show the existence of two types of adsorption (physisorption is preponderant).The chemical quantum parameters confirm the inhibition efficiency of MTNI.Condensed Fukui functions show the nucleophilic and electrophilic sites in the molecule.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML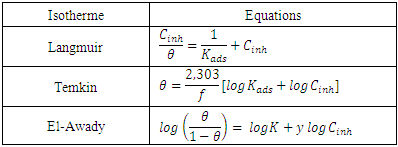
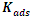 values and thermodynamic adsorption parameters for MTNI
values and thermodynamic adsorption parameters for MTNI