-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
International Journal of Materials and Chemistry
p-ISSN: 2166-5346 e-ISSN: 2166-5354
2021; 11(1): 10-16
doi:10.5923/j.ijmc.20211101.02
Received: Oct. 10, 2021; Accepted: Oct. 27, 2021; Published: Oct. 30, 2021

Synthesis and Characterization of Low Molecular Weight Hyaluronan Sulfates
Khusniddin Khasanbaevich Kirgizbaev1, Bakhtiyor Ikromovich Mukhitdinov1, Abbaskhan Sabirkhanovich Turaev1, Nodirali Sakhobatalievich Normakhamatov2, Dilnoza Mukhtarovna Amonova1, Azizbek Anvarjon Ogli Boydedaev1
1Institute of Bioorganic Chemistry, Academy of Sciences of the Republic of Uzbekistan
2Doctor of Chemical Sciences, Leading Researcher, Tashkent Pharmaceutical Institute
Correspondence to: Khusniddin Khasanbaevich Kirgizbaev, Institute of Bioorganic Chemistry, Academy of Sciences of the Republic of Uzbekistan.
| Email: |  |
Copyright © 2021 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

In this study, the sulfate esterification reactions of low molecular weight hyaluronic acid having degree of polymerization (DP) of 48 with SO3/pyridine (Py) in Py and dimethyl sulfoxide (DMSO) were studied. In the reactions, sulfated hyaluronan samples with degree of substitution (DS) values of 0.22-2.66 were obtainedwith yields of 13.91-78.17%. An increase in the amount of sulfating reagent and the reaction duration in the reaction resulted in an increase in the DS value of the product obtained. In the reactions conducted in DMSO, pronounced polysaccharide chain degradation was observed. The sulfated hyaluronans prepared were structurally characterized by IR- spectroscopic method. Executing the reaction with SO3/Py / Py system (6.0 mol/mol hyaluronic acid unit (HAU) at 80°C for 4 h was found to be suitable for the preparation of sulfated hyaluronans having high DS values.
Keywords: Hyaluronic acid, Sulfation, Sulfated hyaluronic acid, Structure
Cite this paper: Khusniddin Khasanbaevich Kirgizbaev, Bakhtiyor Ikromovich Mukhitdinov, Abbaskhan Sabirkhanovich Turaev, Nodirali Sakhobatalievich Normakhamatov, Dilnoza Mukhtarovna Amonova, Azizbek Anvarjon Ogli Boydedaev, Synthesis and Characterization of Low Molecular Weight Hyaluronan Sulfates, International Journal of Materials and Chemistry, Vol. 11 No. 1, 2021, pp. 10-16. doi: 10.5923/j.ijmc.20211101.02.
Article Outline
1. Introduction
- Extraction of sulfated polysaccharides from natural sources requires many costly processes such as extraction, enrichment, complex purification [1]. Also, the fact that natural sulfated polysaccharides have a complex structure prevents the identification of structural-biological interactions in their study [2, pp. 213-259]. A wide range of biological activities is observed not only in natural sulfated polysaccharides, but also in chemically modified sulfate derivatives of polysaccharides [3]. Sulfated polysaccharides can be obtained by the addition of sulfate groups to polysaccharide macromolecules such as cellulose, dextran, pullulan, hyaluronic acid, chitosan, i.e. by chemical sulfation, and they have a number of advantages over their natural analogues [4,5].
2. The Main Findings and Results
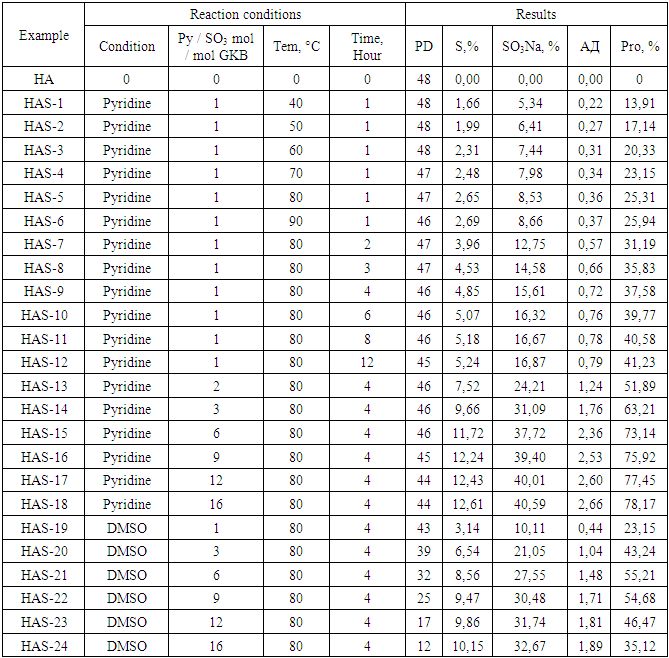
- Currently, hyaluronic acid with different molecular weight (Mw) values is widely used in many fields, including medicine, cosmetology and food industry [6,7]. Today, the delivery of biologically active compounds to the target organs and the detection of non-toxic, easily absorbed in the body molecules specific to cell receptors are among the main directions in the study of drugs. In this regard, hyaluronic acid is a potential vector in the delivery of anti-tumor drugs due to its toxicity, biodegradability in the body and selective binding to CD44 receptors highly expressed in tumor cells [8,9]. However, due to the high Mw value of natural hyaluronic acid (2-6 mDa), it forms viscous solutions. This, in turn, raises problems with the solubility of drug delivery platforms developed on its basis [10,11]. Small molecular weight hyaluronic acid can be used to solve this problem, but its biodegradation time in the presence of hyaluronidases in the body is very short [12]. This leads to a sharp decrease in the efficiency of delivery of anti-tumor drugs of low molecular weight hyaluronic acid. Recent studies have shown that hyaluronic acid sulfate derivatives inhibit hyaluronidase enzymes [13,14]. This suggests that the study of effective methods for the preparation of low molecular weight sulfated hyaluronic acids is relevant. To date, a number of directions of polysaccharide sulfation reactions have been studied [15]. Polysaccharides can be sulfated under heterogeneous, semi-homogeneous and homogeneous conditions depending on the aggregate state of the reaction medium. H3SO4, SO3, HSO3Cl, SO2Cl2 NH2SO3H and SO3 / amine complexes such as SO3 / DMF, SO3 / Py can be used as sulfating reagents [16,17]. In this study, sulfation reactions of a sample of low molecular weight hyaluronic acid with SO3 / Py in a Py (pyridine) and DMSO environment were investigated. In order to determine the optimal reaction conditions, studies were performed with different temperatures, times, and amounts of sulfating reagents. During the reaction, the values of EA (exchange rate) and PD (polymerization rate) of the product, the rate of sulfation, changes in product yield were studied.
3. Materials and Methods
3.1. Sulfation of Hyaluronic acid with Small Molecular Weight
- A solution of low molecular weight hyaluronic acid (Mw = 19.3kDa, PD = 48) at a concentration of 25.0 mg / ml in Py or DMSO was prepared. After complete dissolution of the hyaluronic acid in solution, the reaction mixture was placed in an oil bath and SO3 / Py was added at a ratio of 1.0: 1.0-16.0mol / mol hyaluronic acid unit (GKB) to the amount of hyaluronic acid. Reactions were carried out in Py or DMSO solution at 40–80°S for 1–12 h. Upon completion of the reaction, the solution was cooled to room temperature and neutralized using a 0.1 mol / l solution of NaOH (brought to pH 9). To remove the hyaluronic acid sulfate derivatives from the additives, it was dialyzed using a dialysis membrane (MWCO 1000 Da, Spectrum laboratories, USA) and dried using a lyophilic desiccant.
3.2. Determination of Molecular Mass
- The values and molecular mass sizes of the samples were determined by gel chromatography. Experiments Jasco PU-980 pump (JASCO GmbH, Germany), Suprema 3000 Å and 30 Å columns (PSS Polymer Standards Service GmbH, Germany), SLD7100 MALLS detector (PSS Polymer Standards Service GmbH, Germany), UV-VIS detector (PSS Polymer) Standards Service GmbH, Germany) and a gel chromatography system equipped with a Jasco RI-930 refractive index (RI) detector (JASCO GmbH, Germany). Determination of MM and MM vi sizes was carried out at 25°C and an aqueous solution of 0.1 M NaNO3 was used as an eluent. The columns were calibrated to pullulan standards (Sigma-Aldrich Chemie GmbH, Germany).
3.3. Determination of Sulfur Content
- The sulfur content of the sulfated samples was determined using EURO EA 3000 (CHNS) (O) Elemental Analyzer (EuroVector Instruments & Software, Italy).
3.4. Study of Structures
- The IR spectra of the samples were obtained on a Thermo Nicolet AVATAR 370 FTIR spectrometer, in the absorption range of 400–4000 cm-1, using the KBr tablet method.
4. Results and Their Discussion
- Sulfation reactions of low molecular weight hyaluronic acid using SO3 / Py were performed in Py and DMSO environments. The reaction proceeds by the mechanism of electrophilic exchange between the sulfating reagent and the hyaluronic acid-ON groups. The sulfation reaction of hyaluronic acid with SO3 /Py is shown in Figure 1.
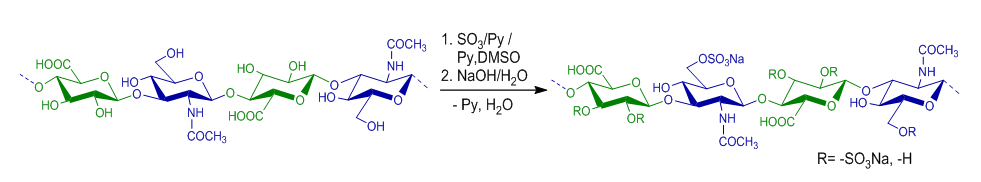 | Figure 1. Sulfation reaction of hyaluronic acid with SO3 / Py in Py or DMSO medium |
|
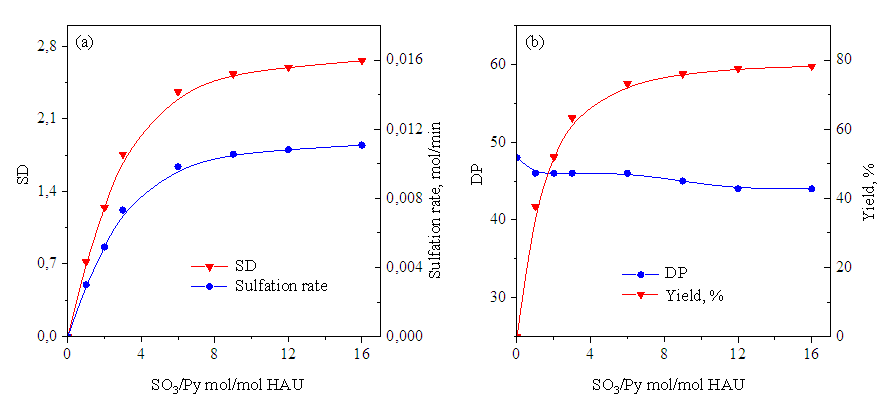
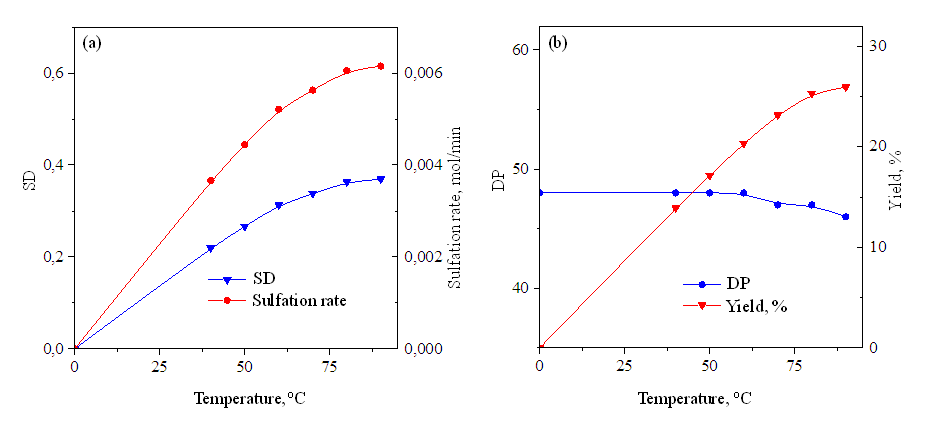 | Figure 2. When sulfating hyaluronic acid with SO3 / Py, changes in ER and sulfation rate (a) and PD and yield (b) under the influence of temperature (SO3 / Py 1.0 mol / mol GKB, time 1 hour) |
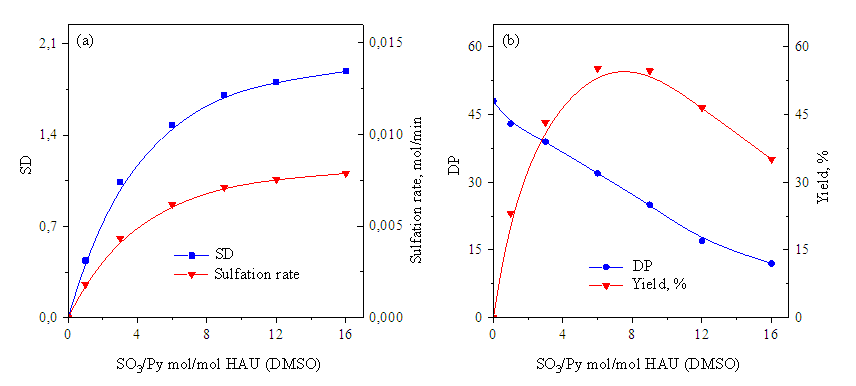
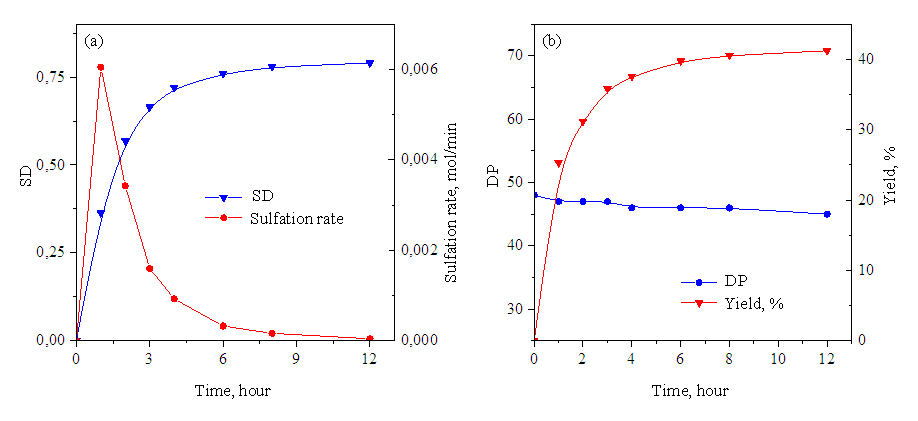 | Figure 3. Changes in ER and sulfation rate (a) and PD and yield (b) during sulfation of low molecular weight hyaluronic acid with SO3 / Py (SO3 / Py 1.0 mol / mol GKB, temperature 80°S) |
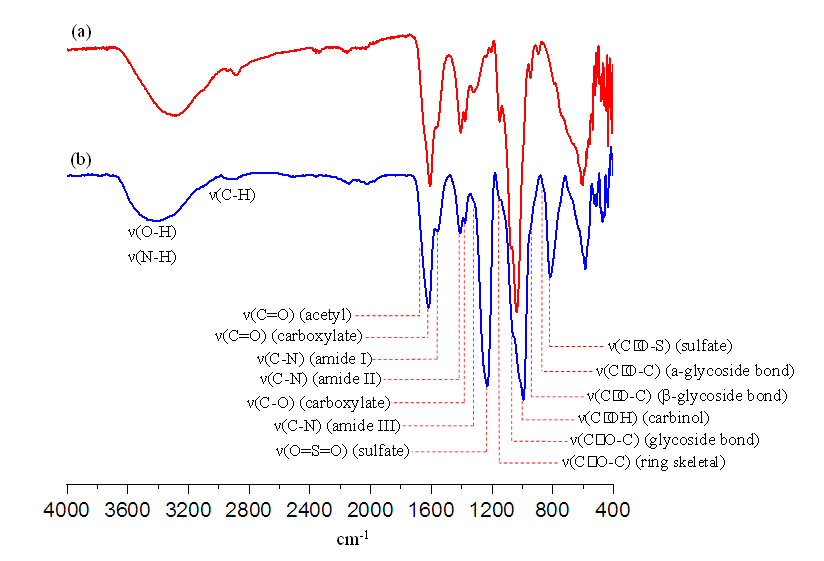 | Figure 6. IR spectra of hyaluronic acid and its sulfated product. (In the spectra: (a) hyaluronic acid Mw = 19.3kDa, PD = 48, PDI = 2.46 (b) NAS-15 Mw = 29.5 kDa, PD = 46) |
5. Conclusions
- Sulfation reactions of low molecular weight hyaluronic acid with SO3 / Py in Py and DMSO environments were studied. It was found that samples with high ER value (ER = 2.66) can be obtained when sulfation reactions are carried out in SO environment with SO3 / Py. An increase in the amount of sulfating reagent in the reaction led to an increase in the ER value of the product obtained as well as the reaction yield. Studies have shown that it is advisable to carry out the sulfation reaction of low molecular weight hyaluronic acid in a Py medium with a sulfating reagent content of 6.0 mol / mol GKB at 80°C for 4 hours. The identified optimal conditions can be used in the preparation of sulfate derivatives of hyaluronic acid with a high ER value of small molecular weight.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML