Alieva G. K.1, Kadirova Sh. A.1, Nuralieva G. A.1, Ashurov J. M.2
1National University of Uzbekistan Named after Mirzo Ulugbek, Tashkent
2Institute of Bioorganic Chemistry, Academy of Sciences of Uzbekistan
Correspondence to: Alieva G. K., National University of Uzbekistan Named after Mirzo Ulugbek, Tashkent.
| Email: |  |
Copyright © 2021 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Abstract
Complex compounds of Cu (II), Zn (II), Ni (II) and Co (II) chloride, nitrate and acetate salts were synthesized on the basis of 1,2,3-benzotriazole derivatives. Composition of synthesized complex compounds, structure element analysis, EQ spectroscopy, RCA, chromato-mass spectrometry, energy-dispersion analysis, electron diffusion reduction spectrum, individuality of newly synthesized complex compounds on the basis of modern quantum chemical calculations and 3d metals of heterocyclic ligands the laws of coordination were determined.
Keywords:
1,2,3-benzotriazole (L1), 1-acetyl-1,2,3-benzotriazole (L2), 2- (2H-benzotriazole-2-il) acetic acid (L3), 2- (1H-Benzotriazole- 1) acetic acid (L4), Chelate, Ligand, Acidoligand, 3d-metal, Thermal Analysis, Composition, Structure, Properties, Complex compound
Cite this paper: Alieva G. K., Kadirova Sh. A., Nuralieva G. A., Ashurov J. M., Synthesis and Study of Complex Compounds with Benzotriazole Products of 3d-Metals, International Journal of Materials and Chemistry, Vol. 11 No. 1, 2021, pp. 1-9. doi: 10.5923/j.ijmc.20211101.01.
1. Introduction
One of the important tasks of modern coordination chemistry is to carry out the directed synthesis of properties-content-structure compounds. This, in turn, requires a systematic study of the relationship between the composition, structure and properties of coordination compounds. By the end of the twentieth century, the chemistry of heterocyclic compounds had become one of the constantly evolving fields of organic chemistry. The use of many nitrogen-containing heterocyclic compounds as highly effective drugs has led to an increase in interest not only in chemists but also in biologists and medical professionals. Nevertheless, the literature on the complex formation of these compounds with intermediate metals is rare.Part of the experiment.In this study, the synthesis of complex compounds of Co (II), Ni (II), Cu (II) and Zn (II) salts on the basis of 1,2,3-benzotriazole derivatives, their spatial structure and "structure-composition-property" correlation were determined.In the study, new complex compounds were synthesized with 1,2,3-benzotriazole and its derivatives of Co (II), Ni (II), Cu (II) and Zn (II) salts. EQ-, PMR-spectroscopy, RCA, chromato-mass spectrometry, energy-dispersion analysis, electron diffusion reduction spectra, the individuality of newly synthesized complex compounds based on modern quantum chemical calculations and the laws of coordination of heterocyclic ligands with 3d-metals were determined.The crystal structure of the synthesized new (L4) chemical compound was determined and entered into the Cambridge Crystallographic Data Center in the United Kingdom (Crystallography Open Database, https: /www.crystallography.net [Cu (BTCK)2 (MEA) 2] CCDC deposit number: 1891272 [1].Thermal analysis was performed on a thermodynamic instrument - Paulik - Paulik - Erdey derivatograph. Simultaneously, the mass of the sample, the decomposition mass of the complexes, and the thermal stability are determined to change with increasing temperature. As a result of thermal analysis, the decomposition and liquefaction of complexes, the coordination quality of ligands determine the final products of the complexes [2,3].
2. Results
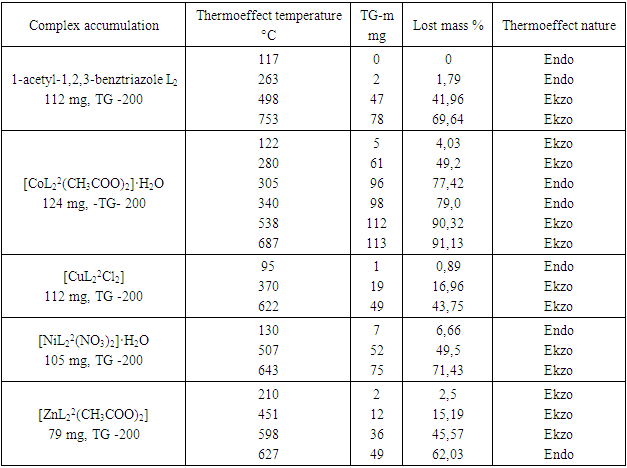
Derivatography were analyzed to determine the thermal stability of complex compounds and the presence of water molecules in their composition. The composition and structure of complex compounds were studied using thermal analysis [4]. The [CuL12(CH3COO)2] 2] complex was analyzed based on derivatographic results. The endothermic effect at 109°C and the exothermic effect at 278, 623, 830°C were determined on the DTA curve of the complex compound containing [CuL12(CH3COO)2]. The first effect corresponds to the decomposition of one molecule of water. Subsequent thermoeffects are explained by the decomposition of the organic ligand and acetate group, as well as the formation of copper oxide, a product of thermolysis (Fig. 1).In the derivatogram of the complex compound containing [СоL12Cl2], endothermic and exothermic effects of 206, 315, 468 and 710°C were observed at 108°C. The first endo-effect represents the decomposition of two molecules of water, from which the total mass loss is 34%. Subsequent thermoeffects characterize the decomposition of an organic molecule in a complex compound. The formation of cobalt (II) chloride as a product of thermolysis has been identified.(L2) 1-acetyl-1,2,3-benzotriazole has endothermic effects at 117 and 263°C on the DTA curve and exothermic effects at 498 and 753°C. The exothermic effects at 498 and 753°C represent the decomposition of the organic ligand (Table 1).[NiL22(NO3)2]•H2O A complex compound containing H2O is characterized by an endothermic effect at 130°C on the DTA curve and exothermic effects at 507 and 643°C. The endothermic effect at 130°C is due to the decomposition of crystallizing water. As the temperature increases, the decomposition of the complex compound increases intensively (Fig. 1). As a result, nitrate begins to break down into ions, hydrazine, nitric oxide, and carbon dioxide. Nickel (II) oxide is formed as a product of thermolysis [4].Table 1. Results of thermal analysis of L2 and complex compounds
 |
| |
|
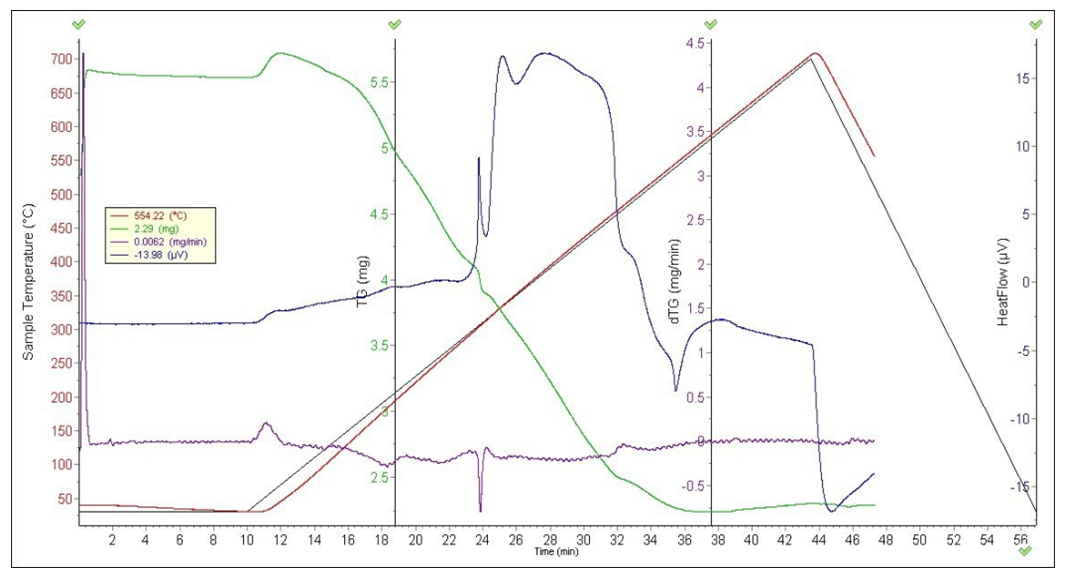 | Figure 1. Derivatogram of the complex compound[СuL12 (CH3COO)2 |
[CoL22(CH3COO)2]•H2O the two endothermic effects 305 and 340°C and the exothermic effects 122, 280, 538 and 687°C are significant on the heating curve of the compound compound. The 122°C exoefficient represents the release of a molecule of water from a complex compound. Cobalt (II) oxide is formed as a product of thermolysis.[CuL22Cl2] The endothermic effect of the complex compound at 95°C and the exothermic effect at 370 and 622°C are significant. Mass reduction at 160-250°C is 4.20%. The decrease in mass in the range 396-458, 458-692°C, which has the nature of exothermic effect, is 83.61%.[ZnL22(CH3COO)2]·H2O. The DTA curve of the complex compound has endothermic properties of 138°C and exothermic effects of 210, 451 and 598°C. The decrease in mass by 32.03% of the total mass is explained by the loss of moisture in the compound. The decrease in mass at 38–144°C is 0.17%, which corresponds to a decrease in one molecule of water. At 168-210°C, the decrease in mass on the TG curve is 18.7%, there are three exothermic effects in the range of 210-370, 370-458, 458-627°C, and the decrease in mass at 627°C is 69.8%.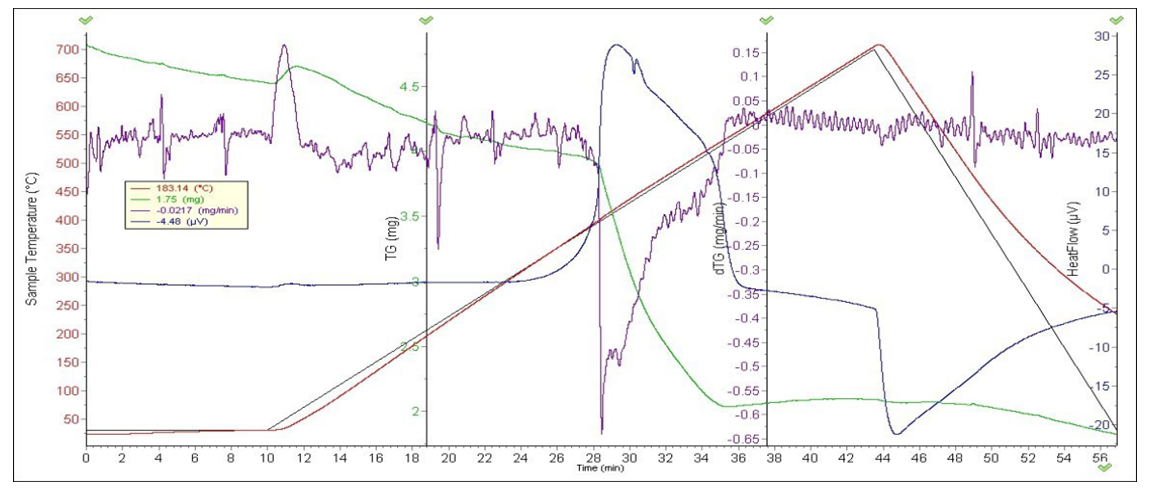 | Figure 2. [NiL22(NO3)2]H2O derivatogram |
Based on the analysis of the results of the study, it was determined that water is located in the outer sphere in the composition of complex compounds. It was concluded that the complex compounds synthesized on the basis of 1-acetyl-1,2,3-benzotriazole are in the form of crystal hydrate.Unlike the 1,2,3-benzotriazole molecule, the 1-acetyl-1,2,3-benzotriazole molecule has the ability to form additional coordination bonds, and the oxygen atom in the acetyl group in the L2 molecule also participates in the formation of the coordination bond.Study of L2 ligand and its complexes by elemental analysis, DTA, chromato-mass spectrometry, EQ- and PMR-spectroscopic methods can be concluded as follows: L2 ligand Co (II), Ni (II), Cu (II) and Zn (II) salts form a coordination bond with the central atom of the second nitrogen atom of the 1-acetyl-1,2,3-benzotriazole ring and the oxygen atom of the acetyl group.In the chromato-mass spectrum of the synthesized compounds, the formation of ions corresponding to their molecular mass was determined. In the analysis using the mass spectrum of complex compounds, the presence of fragmented ions was observed. If the mass of fragmented ions consists of even values, the regrouping process confirms that at odd values, the chemical bonds have a simple breaking process. The presence of metastable ions in the spectrum was investigated. The molecular weight of the complex compounds was determined and the formation of fragmented ions was studied, and a conclusion was drawn about the structure of the substance [5].To determine the relative molecular weight and quantitative study of complex compounds, the samples were analyzed by UECX-mass spectrometry in methanol solution.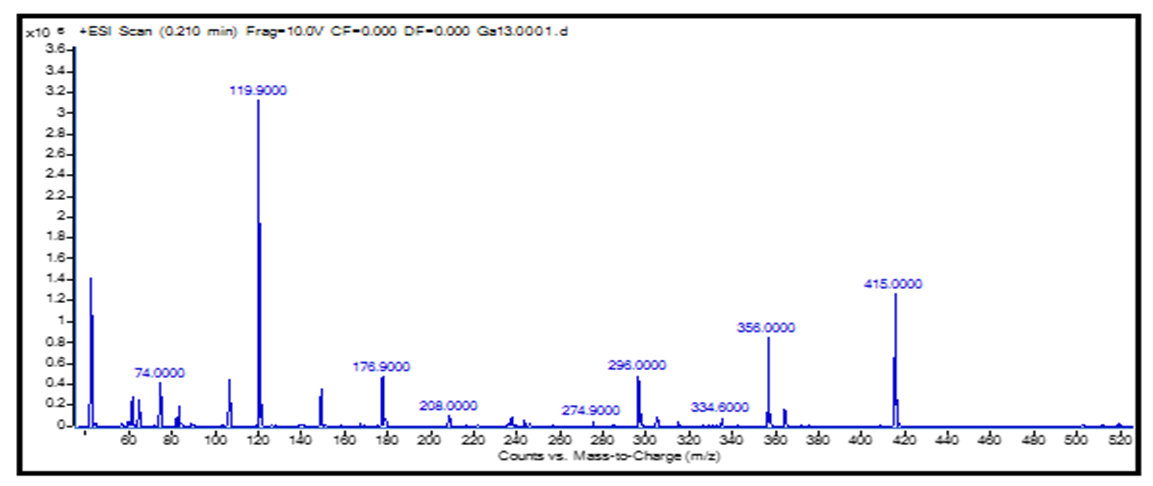 | Figure 3. [CoL12(CH3COO)2]chromato-mass spectra |
[CoL21(CH3COO)2] the decomposition of the complex compound into fragmentary ions formed from molecular ions in the chromato-mass spectrum was determined. ions equal to 415 m / z form ions equal to m / z 356, 334, 296, 274, 208, 176, 119, 74 (Fig. 3). This complex corresponds to the parts belonging to the compound. In the ESI-MS spectrum of a complex of CoL22 (CH3COO) 2, the values of six complex ions were observed in the spectrum: [CoL2 (CH3COO) 2] +, [CoL2 (CH3COO)] +, [CoL2] +, [CoL (CH3COO) 2] +, and [CoL] +. The exact peak in the mass spectrum of a complex compound belongs to the molecular ion [M] + * (m / z = 415). The peak of HL + (m / z = 119.9) is the most intense in the spectrum. As a result, the decomposition phase of M + * led to the decomposition of a single acetate ion in the complex compound, resulting in the formation of a stable ion with m / z = 356. It was then observed that the decomposition of a single ligand molecule from the complex compound results in a stable ion with m / z = 296. In addition, the cleavage of the second ligand molecule was observed, resulting in the formation of cobalt (II) acetate m / z = 176.9.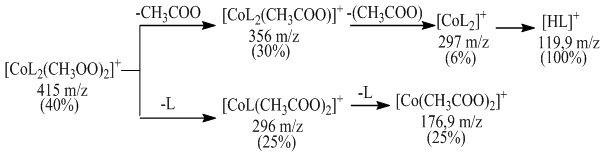 CoL12(CH3COO)2] 2] fragmentation of a complex compound
CoL12(CH3COO)2] 2] fragmentation of a complex compound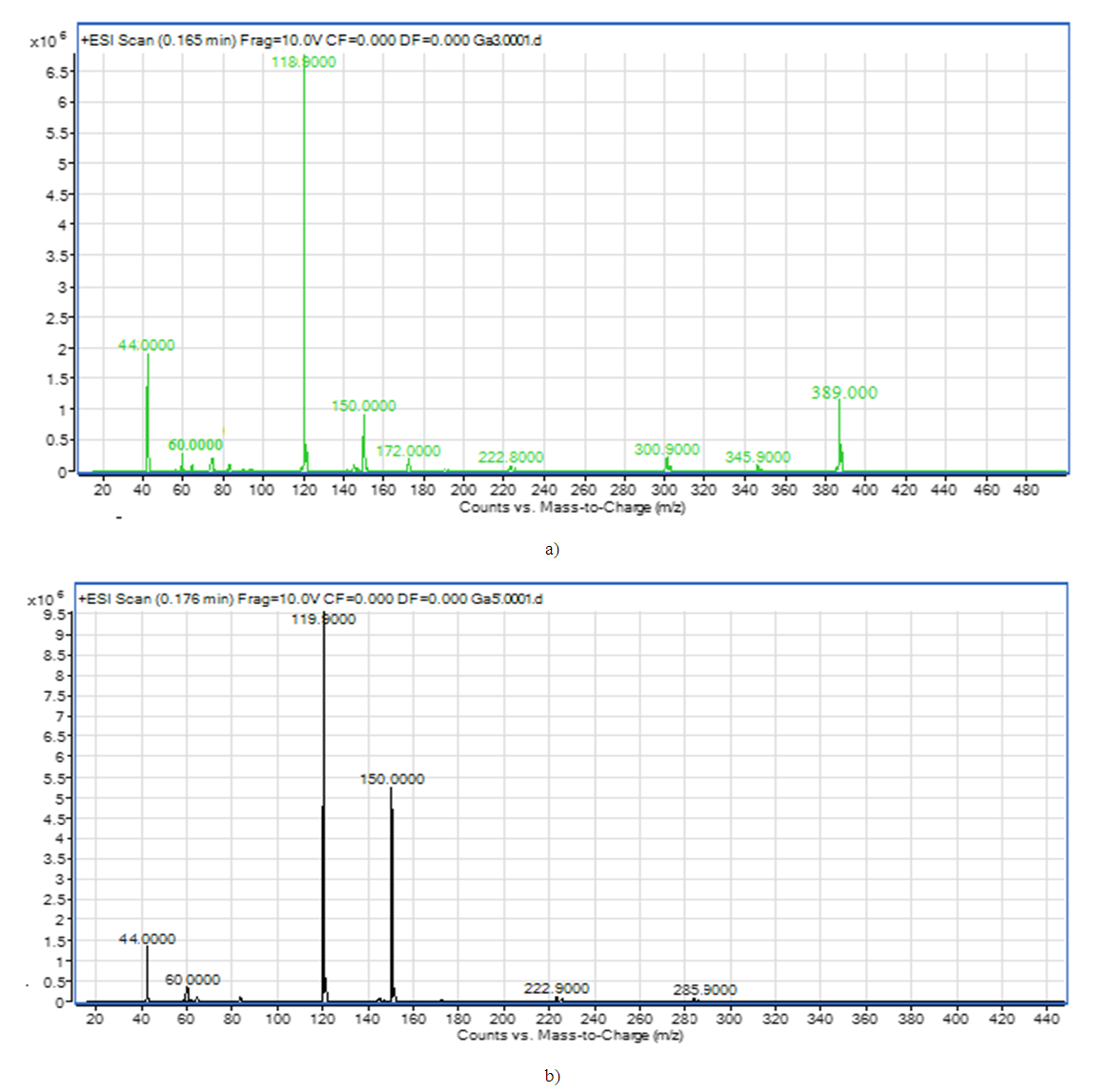 | Figure 4. a) [ZnL24(CH3COO)2] and b) [CuL4МЕА] chromato-mass spectrum |
[CuL22(CH3COO)2]decomposition of a complex compound into fragmentary ions formed from molecular ions in the chromato-mass spectrum. ions equal to m / z 345 form ions equal to m / z 300, 222, 171, 150, 119, 74. The decomposition of a complex compound containing [ZnL24(CH3COO)2] into fragmentary ions formed from molecular ions in the chromato-mass spectrum was found. ions equal to m / z = 216, 172, 149, 119, 74, 42 are formed from ions equal to m / z = 329 [6].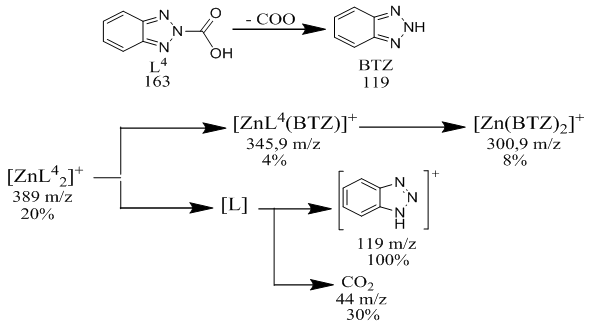 [ZnL24(CH3COO)2] Fragmentation of a complex compound.The decomposition of a complex compound containing [ZnL24(CH3COO)2] into fragmentary ions formed from molecular ions in the chromato-mass spectrum was found. ions equal to m / z = 300, 222, 172, 150, 119, 60, 44 are formed from ions equal to m / z = 389 [7].The decomposition of a complex compound containing CuL4МЕА into fragmentary ions formed from molecular ions in the chromato-mass spectrum was found. ions equal to m / z 285 form ions equal to m / z 222, 150, 119, 60 and 44.
[ZnL24(CH3COO)2] Fragmentation of a complex compound.The decomposition of a complex compound containing [ZnL24(CH3COO)2] into fragmentary ions formed from molecular ions in the chromato-mass spectrum was found. ions equal to m / z = 300, 222, 172, 150, 119, 60, 44 are formed from ions equal to m / z = 389 [7].The decomposition of a complex compound containing CuL4МЕА into fragmentary ions formed from molecular ions in the chromato-mass spectrum was found. ions equal to m / z 285 form ions equal to m / z 222, 150, 119, 60 and 44.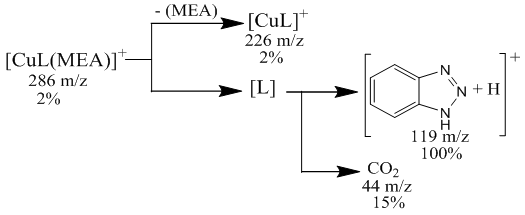 [CuL4MEA] Fragmentation of a complex compoundIn the chromato-mass spectra of complex compounds, peaks of initial and final ions, characteristic of metastable transitions, were observed. Molecular structure was determined by determining the initial and final masses of metastable ions [8].The structure of complex compounds was analyzed using EQ spectrum. L4 (2- (2N-benzotriazole-2-il) acetic acid) and metal salts were synthesized in ethanol solution in the ratio L: M 2: 1 with complex compounds containing МL24·H2O, L4 and monoethanolamine andcomplex compounds with ML4 · MEA content in the ratio L: M: L 1: 1: 1 in ethanol solution with salts of metals were synthesized. L4- 2- (2N-benzotriazole-2-il) acetic acid and monoethanolamine. EQ- spectra of all synthesized complex compounds were analyzed to determine the coordination center of the polyfunctional polydanate ligand (Table 2).
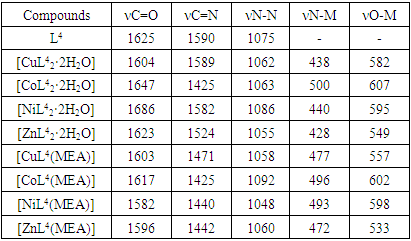
[CuL4MEA] Fragmentation of a complex compoundIn the chromato-mass spectra of complex compounds, peaks of initial and final ions, characteristic of metastable transitions, were observed. Molecular structure was determined by determining the initial and final masses of metastable ions [8].The structure of complex compounds was analyzed using EQ spectrum. L4 (2- (2N-benzotriazole-2-il) acetic acid) and metal salts were synthesized in ethanol solution in the ratio L: M 2: 1 with complex compounds containing МL24·H2O, L4 and monoethanolamine andcomplex compounds with ML4 · MEA content in the ratio L: M: L 1: 1: 1 in ethanol solution with salts of metals were synthesized. L4- 2- (2N-benzotriazole-2-il) acetic acid and monoethanolamine. EQ- spectra of all synthesized complex compounds were analyzed to determine the coordination center of the polyfunctional polydanate ligand (Table 2).Table 2. Basic oscillations of EQ-spectra of L4 and complex compounds frequencies (cm-1)
 |
| |
|
The -C = N-, N-N, C-N, COO- functional groups in the acetic acid molecule 2- (2H-benzotriazole-2-il) are of significant importance and exhibit significant valence oscillations in the EQ spectrum.In the EQ spectra of the complexes, it was observed that the absorption lines corresponding to the triazole ring -C = N and COO- groups changed to a short field. In the spectra of the complexes, absorption lines 549-607 cm-1 and 428-500 cm-1 corresponding to the valence oscillations of the O-M and N-M bonds are observed in the short wavelength range [9]. The vibrations of the benzene ring belonging to the -CH group remain unchanged and are located in the area of 2939-2992 cm-1. This also confirms the results of quantum chemical calculations performed on a ligand molecule [10].2- (2H-benzotriazole-2-il) was carried out in the presence of additional ligand monoethanolamine in order to increase the chemical activity of acetic acid and to study the possibility of complex formation.The valence oscillations of monoethanolamine-C-ON were 3450 cm-1, and the deformation oscillations of the NH-group were 1120 cm-1 [11]. In the complex compounds, however, 3310 and 1069 cm-1 areas were identified, indicating the formation of a chelated coordination bond with the MEA.In the EQ spectrum of complex compounds, it was observed that the valence oscillations of the C = N bond, in contrast to the ligand, shifted to ≈80-120 cm-1, the valence oscillations of the COO-bond shifted to a short area ≈25-31 cm-1. In the EQ spectrum of complex compounds, N-M and O-M corresponding valence oscillations were observed in the 472-496 and 533-602 cm-1 regions. This means that it is coordinated with the metal by the nitrogen and oxygen atoms in the L4 molecule. When analyzed using modern physical research methods, it was observed that monoetonolamine bidate forms in coordination with the metal atom.When analyzing the structure of complex compounds, the stability of the N-M and O-M bond and the bond energy decrease according to the Co> Ni> Cu> Zn series, which is consistent with the literature. The stability of complex compounds formed by divalent metals is consistent with Irving-Williams law [12].The structure of 2- (2H-benzotriazole-2-il) acetic acid (L4) was analyzed using RCA. 2- (2H-benzotriazole-2-il) A new polymorphic form of acetic acid, C8H7N3O2, crystallizes in the spatial C2 / c (Z = 8) group, in monoclinic syngony. The carboxyl group of this uneven molecule has a sinplanar conformation. Crystal structure spirals have O1-H7N3 intermolecular hydrogen bonds that run continuously along the b axis of the crystal cell. However, in the given structure, benzotriazole fragments are located symmetrically to the center and are stacked in a certain sequence with neighboring molecules [centroid-centroid distance = 3,593 (10) and 3,381 (10) Å], while in other polymorphs, such stacking is not observed. 2- (1H-benzotriazole-1-il) acetic acid crystals were also observed in C7-H6O2 bonds [13,14,15].2- (2H-benzotriazole-2-il) acetic acid in a certain polymorphic form (polymorphic I) molecules have strong O1-H7 ∙∙∙ N3 hydrogen bonds located parallel to the b-axis in a spiral [D ∙∙∙ A = 2.6995 Å; angle D-H ∙∙∙ A = 168.08 °]. Crystals of a new polymorphic form of acetic acid 2- (1N-benzotriazole-1-il) belong to the spatial group C2 / c. In an asymmetric unit, uneven free molecules are arranged by a synplanar conformation of carboxyl groups (Fig. 5). The carboxyl groups were deviated from the plane of the 1,2,3-benzotriazole fragment at an angle of 88.41 (15) ° (C1 / C2 / C3 / C4 / C5 / C6 / N1 / N2 / N3). The spirals formed O1-H7 ∙∙∙ N3 hydrogen bonds between molecules parallel to the b-axis [D ∙∙∙ A = 2.7273 (17) A; D-H ∙∙∙ A = 171 °], in this respect they are polymorphic [16,17].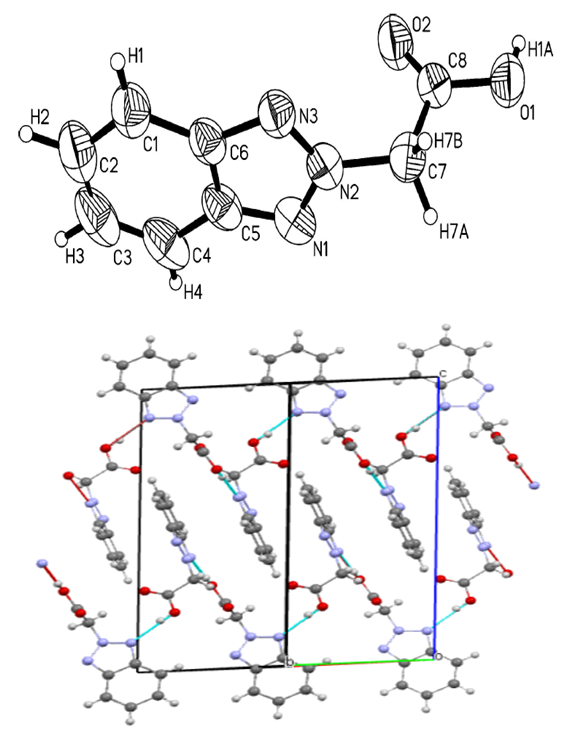 | Figure 5. Crystal structure of 2- (2H-benzotriazole-2-il) acetic acid |
Benzotriazole fragments are located in the center of symmetry with respect to adjacent molecules and are folded with a p-p effect [centroid-centroid (1.5 - x, 0.5 - y, -z) distance = 3.593 (10) Å, C6 ∙∙∙ C6 (1.5 - x, 0.5 - y, -z) distance 3.381 (10) Å] and [C6 ∙∙∙ C2 (1 - x, 1 - y, -z) distance 3.361 (10) Å]. No folding effects were observed in the known I polymorph. The crystal structure of 2- (2H-benzotriazole-2-il) acetic acid was stabilized due to additional C-H ∙∙∙ O effects [C2 ∙∙∙ O2 = 3.365 (3) Å; angle C2-H2 ∙∙∙ O2 = 148 °, C7 ∙∙∙ O1 = 3.387 (3) Å; angle C7-H7A ∙∙∙ O1 = 154 °, C7 ∙∙∙ O2 = 3.268 (3) Å; angle C7-H7B ∙∙∙ O2 = 150 °].(1H-Benzotriazole-1-il) acetic acid (HBTCK; C8H7N3O2) and monoethanolamine (MEA; C2H7NO) Cu (II) and [Cu (C8H6N3O2) 2 (C2H7NO) 2] complex compound single-crystal . In its asymmetric unit, one BTCK anion is coordinated with the Cu2 + cation with a carboxyl O atom, and one MEA ligand is bound to a metal cation with two atoms (O and N) to form a chelate. The equatorial Cu-O3 and Cu-N4 bond lengths are 2,029 (1) and 1,980 (2) Å, respectively, and the axial Cu-O2 bond lengths are significantly larger [2,492 (2) Å], which belongs to the Yan – Teller deviation type. Intramolecular hydrogen bonds were formed between the MEA ligand hydroxyl group and the O1 atom that was not involved in the coordination of the carboxyl group. The intermolecular hydrogen bonds formed between the MEA ligand amino group and the carboxyl group formed the eight-membered ring R22 (8). The intermolecular triazole ring and the methylene groups of MEA cross-linked through C6 (B) -H bonds to form a three-dimensional supramolecular framework [18].Internal molecular hydrogen bonds are shown in the form of dashed lines. The displacement of the ellipsoids is described with a 25% probability. The Cu-O3 bond length is 2,492 (2) A °, described as the Yan-Teller deviation type.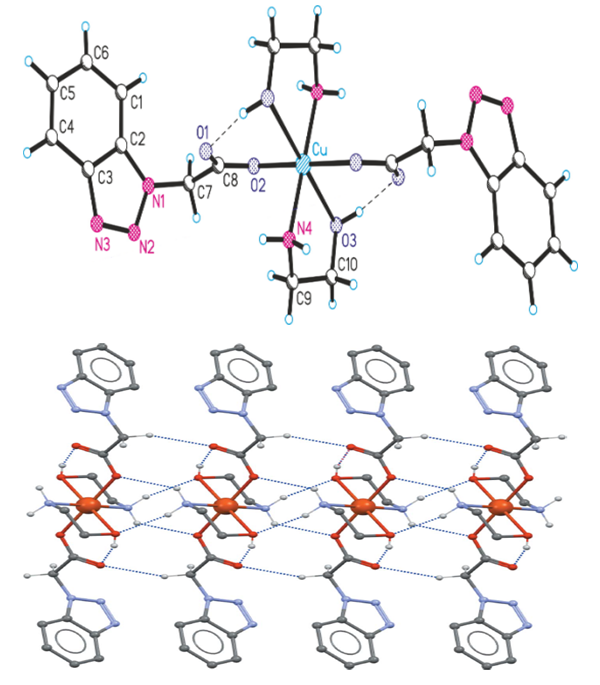 | Figure 6. [Cu (BTCK)2 (MEA 2] The structure of a complex compound |
The MEA ligand is neutral in nature, coordinated by bidentant N and O-donor atoms, and forms a chelate ring in the five-membered twist conformation of CuNC2O; O3-C10-C9-N4 torsion angle 60.3 (3)°. The flat ring system of benzotriazole (N1 – N3 / C1 – C6: deviation = 0.0064 Å) is copied by the methyl group to the C7 atom [deviation from the plane 0.158 (2) Å], while the carboxyl group is almost perpendicular to this plane [88.0 (2)°] (Fig. 6) [19,20].Table 3. Basic crystallographic data of [Cu (BTCK) 2 (MEA) 2]
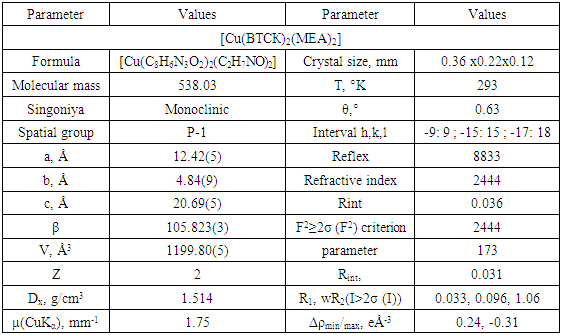 |
| |
|
The difference between the distances of the carboxyl group C8-O (1,2) is (B = 0.036 A °) and indicates that the monodentant is coordinated, the long C-O distance also includes the O2 atom in the coordination. The molecular structure is stabilized by an intramolecular O3-H3O1 hydrogen bond between the OH group of the MEA ligand and the O atom of the uncoordinated carboxyl.Additional ligand monoethanolamine was added to increase the chemical activity and coordination potential of 2- (1H-benzotriazole-1-il) -acetic acid (L3) and 2- (2H-benzotriazole-2-il) acetic acid (L4). The structure, properties and composition of mixed ligand complexes were analyzed as a result of physicochemical studies.Renenstructural analysis revealed that two molecules of the heterocyclic ligand L3 are bonded in the form of a metal salt by an ionic bond, and with an additional monoethanolamine ligand in the form of a bidentate with the metal atom to form an octahedral structure.The structure of complex compounds formed with 3d-metals as a result of physicochemical studies is recommended as follows: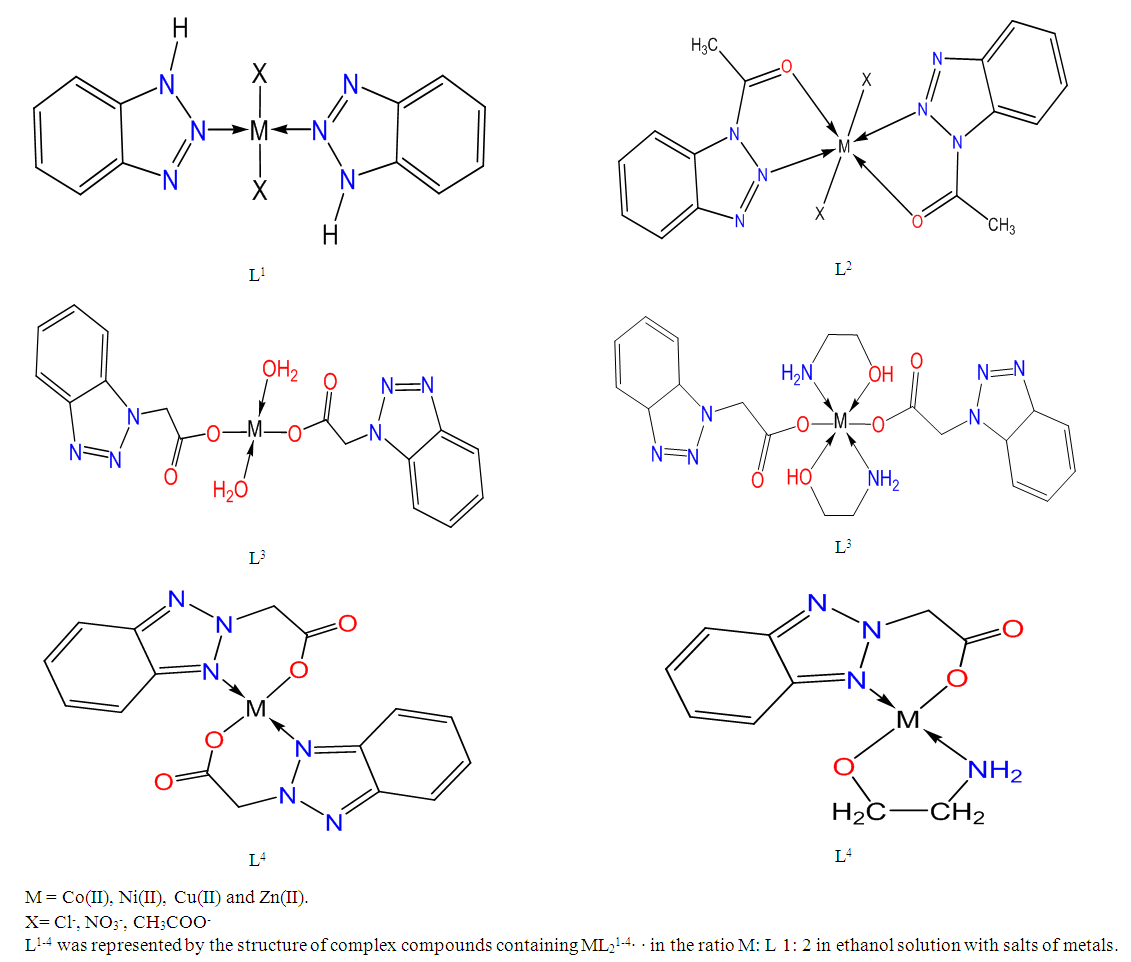 | Figure |
References
| [1] | Alieva G. K. Structure of trans-bis (monoethanolamine) bis (2-1 (H-benzotriazole-1-yl) acetic acid) copper (II) Acta Cryst. 2019. 2019. E 75. 233-236. |
| [2] | Gabbott P. (ed) Principles and Applications of Thermal Analysis. Singapore, Wiley-Blackwell, 2008. 480 p. |
| [3] | Topor N.D., Ogorodova L.P., Melchakova L.V. Thermal analysis of minerals and inorganic compounds. - M .: Izd-vo MGU, 1987. -190 p. |
| [4] | Hokelek T., Necefoglu H. Crystal structure of [triaqua (salicylate) (nicotinamide) zinc (II)] Analytical Sciences, 2001, vol. 17, no. 10, pp. 1241-1142. |
| [5] | V. A. Vinarskiy, R. A. Yurchenko. Mass spectrometry and chromato-mass spectral analysis: posobie / http://elib.bsu.by/handle/123456789/91988. |
| [6] | Zaikin V.G.Osnovy mass-spectrometry of organic compounds // M .: MAIK "Nauka / Interperiodika", 2001. - 286 p. |
| [7] | Liver E.B. Electron spectroscopy of inorganic compounds: Per. s angl. - M .: Mir, 1987. -T.1.-491 c. |
| [8] | Liver E.B. Electron spectroscopy of inorganic compounds: Per. s angl. - M .: Mir, 1987. -T.2. -. 443 c. |
| [9] | Byokker Yu. Spectroscopy. Moscow: Technosphere, 2009. 528 c. ISBN 978-5-94836-220-5. |
| [10] | Tarasevich B.N. IR spectra of the main classes of organic compounds. Spravochnye materials. Moscow. -2012. 55 c. |
| [11] | Kazitsina L.A., Kupletskaya N.B. Application of UF-, IK- and YaMR-spectroscopy in organic chemistry. M.: Kniga po Trebovaniyu. -2013. -264 p. |
| [12] | Nakamoto K. IK - spectra of inorganic and coordination compounds. M .: Mir. 1996. 204 p. |
| [13] | Sheldrick, G. M. (2008). Acta Cryst. A64, 112–122. |
| [14] | Yu, K. L., Zhang, Y., Civiello, R. L., Kadow, K. F., Cianci, C., Krystal, M. &. |
| [15] | Meanwell, N. A. (2003). Bioorg. Med. Chem. Lett. 13, 2141–2144. |
| [16] | Giordano & Zagari (1978). J. Chem. Soc. Perkin Trans. 2, pp. 312–315. |
| [17] | Qu Y., Liu Z.-D., Zhu H.-L., Tan M.-Y. Hexaaquanickel (II) bis (p-nitrobenzoate) dihydrate // Acta Crystallogr. -2004.- E60. P. m1306-m1307. |
| [18] | Oxford Diffraction (2009). CrysAlis PRO. Oxford Diffraction Ltd, Yarnton, England. |
| [19] | G. Alieva, J. Ashurov, N. Mukhamedov. N. Parpiev. A new polymorph of 2- (2H-benzotriazole-2-yl) acetic acid / // Acta Cryst. -2012. -E68. 2833. |
| [20] | Ibragimov A.B., Englert U., Ashurov J.M., Wang A. Dimorphism of the salt hexaaquanickel (II) bis (p-nitrobenzoate) dihydrate: a new triclinic crystal form // J. Str. Chem. In press, 2018. |





 CoL12(CH3COO)2] 2] fragmentation of a complex compound
CoL12(CH3COO)2] 2] fragmentation of a complex compound
 [ZnL24(CH3COO)2] Fragmentation of a complex compound.The decomposition of a complex compound containing [ZnL24(CH3COO)2] into fragmentary ions formed from molecular ions in the chromato-mass spectrum was found. ions equal to m / z = 300, 222, 172, 150, 119, 60, 44 are formed from ions equal to m / z = 389 [7].The decomposition of a complex compound containing CuL4МЕА into fragmentary ions formed from molecular ions in the chromato-mass spectrum was found. ions equal to m / z 285 form ions equal to m / z 222, 150, 119, 60 and 44.
[ZnL24(CH3COO)2] Fragmentation of a complex compound.The decomposition of a complex compound containing [ZnL24(CH3COO)2] into fragmentary ions formed from molecular ions in the chromato-mass spectrum was found. ions equal to m / z = 300, 222, 172, 150, 119, 60, 44 are formed from ions equal to m / z = 389 [7].The decomposition of a complex compound containing CuL4МЕА into fragmentary ions formed from molecular ions in the chromato-mass spectrum was found. ions equal to m / z 285 form ions equal to m / z 222, 150, 119, 60 and 44. [CuL4MEA] Fragmentation of a complex compoundIn the chromato-mass spectra of complex compounds, peaks of initial and final ions, characteristic of metastable transitions, were observed. Molecular structure was determined by determining the initial and final masses of metastable ions [8].The structure of complex compounds was analyzed using EQ spectrum. L4 (2- (2N-benzotriazole-2-il) acetic acid) and metal salts were synthesized in ethanol solution in the ratio L: M 2: 1 with complex compounds containing МL24·H2O, L4 and monoethanolamine andcomplex compounds with ML4 · MEA content in the ratio L: M: L 1: 1: 1 in ethanol solution with salts of metals were synthesized. L4- 2- (2N-benzotriazole-2-il) acetic acid and monoethanolamine. EQ- spectra of all synthesized complex compounds were analyzed to determine the coordination center of the polyfunctional polydanate ligand (Table 2).
[CuL4MEA] Fragmentation of a complex compoundIn the chromato-mass spectra of complex compounds, peaks of initial and final ions, characteristic of metastable transitions, were observed. Molecular structure was determined by determining the initial and final masses of metastable ions [8].The structure of complex compounds was analyzed using EQ spectrum. L4 (2- (2N-benzotriazole-2-il) acetic acid) and metal salts were synthesized in ethanol solution in the ratio L: M 2: 1 with complex compounds containing МL24·H2O, L4 and monoethanolamine andcomplex compounds with ML4 · MEA content in the ratio L: M: L 1: 1: 1 in ethanol solution with salts of metals were synthesized. L4- 2- (2N-benzotriazole-2-il) acetic acid and monoethanolamine. EQ- spectra of all synthesized complex compounds were analyzed to determine the coordination center of the polyfunctional polydanate ligand (Table 2).


 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML

