| [1] | Techno without borders, copper in all its states, Technology, 2008, 155, 8-13. |
| [2] | Mendonca, G.L.F., Costa, S.N., Freire, V.N., Casciano, P.N.S., Correia, A.N., 2017, Lima-Neto, P.d. Understanding the corrosion inhibition of carbon steel and copper in sulphuric acid medium by amino acids using electrochemical techniques allied to molecular modelling methods, Corrosion Science, 115, 41–55. |
| [3] | Zhang, D.Q., Cai, Q.-R., Gao, L.-X., Lee, K.Y., 2008, Effect of serine, threonine and glutamic acid on the corrosion of copper in aerated hydrochloric acid solution, Corrosion Science. 50(12), 3615–3621. |
| [4] | Laggoun, R., Mahmoud F., Saidat, B., Benghia, A., Chaabani, A., 2020, Effect of p-toluenesulfonyl hydrazide on copper corrosion in hydrochloric acid solution. Corrosion Science 165, 108363. |
| [5] | Talebian, M., Raeissi, K., Atapour, M., Fernández-Pérez, B.M., Salarvand, Z., Meghdadi, S., Amirnasr, M., Souto, R.M., 2018, Inhibitive effect of sodium (E)-4-(4-nitrobenzylideneamino) benzoate on the corrosion of some metals in sodium chloride solution. Applied Surface Science 447, 852-865. |
| [6] | Qiang, Y., Zhang, S., Xu, S., Li, W., 2016, Experimental and theoretical studies on the corrosion inhibition of copper by two indazole derivatives in 3.0% NaCl solution, Journal of colloid and interface science, 472, 52-59. |
| [7] | Wang, D., Xiang, B., Liang, Y., Song, S., Liu, C., 2014, Corrosion control of copper in 3.5wt.% NaCl Solution by Domperidone: Experimental and Theoretical Study, Corrosion Science 85,77-86. |
| [8] | Saira, F., Renu, S., Faiza A., Ajar K., Amin, B., Heinz-Bernhard, K., 2019) Study of new amphiphiles based on ferrocene containing thioureas as efficient corrosion inhibitors: Gravimetric, electrochemical, SEM and DFT studies. Journal of Industrial and Engineering Chemistry 76, 374-387. |
| [9] | Döner, A., Yüce, A.O., Kardaş, G., 2013, Inhibition Effect of Rhodanine-N-Acetic Acid on Copper Corrosion in Acidic Media, Industrial & Engineering Chemistry Research 52(29), 9709-9718. |
| [10] | L. Gao, S. Peng, X. Huang, Z. Gong,2020, A combined experimental and theoretical study of papain as a biological eco-friendly inhibitor for copper corrosion in H2SO4 medium, Applied Surface Science, 511, 145446. |
| [11] | Tigori, M.A., Bony, F. N., Niamien, P. M., Yapo, A. J., Trokourey, A., 2016, Experimental and theoretical studies on Riboflavin’s behaviour against copper corrosion in 1M HNO3, Archives of Applied Science Research, 8 (5): 18-32. |
| [12] | Singh, A.K., Mohapatra, S. and Pani, B., 2016, Corrosion Inhibition Effect of Aloe Vera gel: Gravimetric and Electrochemical Study. Journal of Industrial and Engineering Chemistry, 25, 288-297. |
| [13] | Deyab, M.A., 2015, Egyptian Licorice Extract as a Green Corrosion Inhibitor for Copper in Hydrochloric Acid Solution. Journal of Industrial and Engineering Chemistry, 25, 384-389. |
| [14] | Ahmed, R.A., 2016, Investigation of Corrosion Inhibition of Vitamins B1 and C on Mild Steel in 0.5 M HCl Solution: Experimental and Computational Approach. Oriental Journal of Chemistry, 32(1), 295-304. |
| [15] | Fucks-Godec, R. and Zergav, G., 2015, Corrosion Resistance of High-Level Hydrophobic Layers Combination with Vitamin E-(α-tocopherol) as Green Inhibitor. Corrosion Science, 97, 7-16. |
| [16] | Zohreh Parsaee, Pouya Haratipour, Milad Janghorban Lariche and Arash Vojood., 2018, A novel high performance nano chemosensor for copper (II) ion based on an ultrasound-assisted synthesized diphenylamine-based Schiff base: Design, fabrication and density functional theory calculations. Ultrasonics Sonochemistry, 41, 337-349. |
| [17] | Elias, E., Elemike T., Henry, U., Nwankwo, D., Onwudiwe C, Hosten, E. C., 2017, Synthesis, crystal structures, quantum chemical studies and corrosion inhibition potentials of 4-(((4-ethylphenyl)imino)methyl)phenol and (E)-4-((naphthalen-2-ylimino)methyl)phenol Schiff bases. Journal of Molecular Structure, 1147, 252-265. |
| [18] | L. Guo, W.P. Dong, S.T. Zhang, 2014, Theoretical challenges in understanding the inhibition mechanism of copper corrosion in acid media in the presence of three triazole derivatives, Royal Society of Chemistry Adv, 4, 41956-41967. |
| [19] | Tigori, M.A., Kouyate, A., Kouakou, V., Niamien, P.M. and Trokourey, A., 2020, Inhibition Performance of Some Sulfonylurea on Copper Corrosion in Nitric Acid Solution Evaluated Theoretically by DFT Calculations, Open Journal of Physical Chemistry, 10(3), 139-157. |
| [20] | I.B. Obot, D.D. Macdonald and Z.M. Gasem., 2015, Density functional theory (DFT) as a powerful tool for designing new organic corrosion inhibitors. Part 1: An overview. Corrosion Science 99, 1-30. |
| [21] | H. Elmsellem, T. Harit, A. Aouniti, F. Malek, A. Riahi, A. Chetouani and B. Hammouti., 2015, Adsorption properties and inhibition of mild steel corrosion in 1 M HCl solution by some bipyrazolic derivatives: Experimental and theoretical investigations. Protection of Metals and Physical Chemistry of Surfaces 51:5, 873-884. |
| [22] | S. John, J. Joy, M. Prajila and A. Joseph., 2011, Electrochemical, quantum chemical, and molecular dynamics studies on the interaction of 4-amino-4H,3,5-di(methoxy)-1,2,4-triazole (ATD), BATD, and DBATD on copper metal in 1N H2SO4. Materials and Corrosion 62(11), 1031-1041. |
| [23] | Karelson, M.; Lobanov, V.S., 1996, Quantum chemical descriptors in QSAR/QSPR studies. Chemical Reviews, 96(3), 1027-1043. |
| [24] | Vera, L.; Guzman, M.; Ortega-Luoni, Y.P., 2006, QSPR study of corrosion inhibitors; imidazolines, Journal of the Chilean Chemical Society, 51(4), 1034-1039(2006). |
| [25] | M. J. Frisch, G. W. Trucks, H. B. Schlegel, G. E. Scuseria, M. A. Robb, J. R. Cheeseman, G. Scalmani, V. Barone, B. Mennucci, G. A. Petersson, H. Nakatsuji, M. Caricato, X. Li, H. P. Hratchian, A. F. Izmaylov, J. Bloino, G. Zheng, J. L. Sonnenberg, M. Hada, M. Ehara, K. Toyota, R. Fukuda, J. Hasegawa, M. Ishida, T. Nakajima, Y. Honda, O. Kitao, H. Nakai, T. Vreven, J. A. Montgomery, Jr., J. E. Peralta, F. Ogliaro, M. Bearpark, J. J. Heyd, E. Brothers, K. N. Kudin, V. N. Staroverov, R. Kobayashi, J. Normand, K. Raghavachari, A. Rendell, J. C. Burant, S. S. Iyengar, J. Tomasi, M. Cossi, N. Rega, J. M. Millam, M. Klene, J. E. Knox, J. B. Cross, V. Bakken, C. Adamo, J. Jaramillo, R. Gomperts, R. E. Stratmann, O. Yazyev, A. J. Austin, R. Cammi, C. Pomelli, J. W. Ochterski, R. L. Martin, K. Morokuma, V. G. Zakrzewski, G. A. Voth, P. Salvador, J. J. Dannenberg, S. Dapprich, A. D. Daniels, Ö. Farkas, J. B. Foresman, J. V. Ortiz, J. Cioslowski and A. D. J. Fox, Gaussian, Inc., Wallingford, (2009), 09. |
| [26] | Benhiba F., Serrar H., Hsissou R., Guenbour A., Bellaouchou A., Tabyaoui M., Boukhris S., Oudda H., Warad I. and Zarrouk A., 2020, Tetrahydropyrimido-Triazepine derivatives as anti-corrosion additives for acid corrosion: Chemical, electrochemical, surface and theoretical studies. Chemical Physics Letters 743, 137181. |
| [27] | C. Lee, W. Yang, R.G. Parr, 1988, Development of the Colle-Salvetti correlation-energy formula into a functional of the electron density, Physical Review B, 37,785-789. |
| [28] | Lukovits, I., Kalman, E.F., 2001, Corrosion Inhibitors — Correlation between Electronic Structure and Efficiency, Corrosion (NACE), 57(1): 3-8. |
| [29] | J. Aljourani, K. Raessi, M. A. Golozar, 2006, Benzimidazole and its derivatives as corrosion inhibitors for mild steel in 1M HCl, Corrosion science, 51, 1836-1843. |
| [30] | J. O’M. Bockris, D. Drazi, 1962; The kinetics of deposition and dissolution of iron: Effect of alloying impurities, Portugaliae Electrochemica Acta, 7, 293-313. |
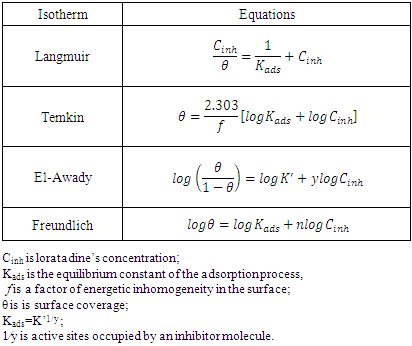
| [31] | M. I. Temkin, 1941, Adsorption equilibrium and process Kinetics on inhomogeneous surfaces with interaction between adsorbed molecules, Zh. Fiz. Khim, 15(3), 296-332. |
| [32] | Y. A. El Awady, A. I. Ahmed, 1985, Effect of temperature and inhibitors on the corrosion of aluminium in 2N HCl solution, A kinetic study, Journal of Indian Chemistry, 24, 601-606. |
| [33] | Irving Langmuir, 1916, the constitution and fundamental properties of solids and liquids, Journal of the American Chemical Society, 38(11), 2221–2295. |
| [34] | Villamil R F. V., Corio P., Rubin J. C., Agostinho S. M. L., 1999, Effect of sodium dodecylsulfate on copper corrosion in sulfuric acid media in the absence and presence of benzotriazole, Journal of Electroanalytical Chemistry, 472, 112-116. |
| [35] | Vashi R. T., Champaneri V. A., 1997, Toluidines as corrosion inhibitors for zinc in sulphamic acid Indian Journal of Chemical Technology, 4,180-184. |
| [36] | Noor E.A., Al-Moubaraki A.H., 2008, Thermodynamic study of metal corrosion and inhibitor adsorption processes in mild steel/1-methyl-4[4(-X)-styryl pyridinium iodides/ hydrochloric acid systems, Materials Chemistry and Physics, 110, 145-154. |
| [37] | F. Bentiss, M. Lebrini, M. Lagrenee, 2005, Thermodynamic characterization of metal dissolution and inhibitor adsorption processes in mild steel/2,5-bis(n-thienyl)-1,3,4-thiadiazoles/ hydrochloric acid system, Corrosion Science, 47, 2915-2931. |
| [38] | J. D. Talati and D. K. Gandhi, 1983, N-heterocyclic compounds as corrosion inhibitors for aluminium-copper alloy in hydrochloric acid, Corrosion Science, 23(12), 1315–1332. |
| [39] | W. Durnie, R. De Marco, A. Jefferson, and B. Kinsella, 1999, Development of a structure-activity relationship for oil field corrosion inhibitors, Journal of the Electrochemical Society, 1999, volume 146(5), 1751–1756. |
| [40] | I.N. Putilova, S. A. Balezin, V. P. Barannik, Metallic Corrosion Inhibitors, Pergamon Press, Oxford, 1960, 30. |
| [41] | S O Adejo; M. M Ekwenchi. IOSR., 2014, Resolution of adsorption characterisation ambiguity through the Adejo-Ekwenchi adsorption isotherm: a case study of leaf extract of Hyptis suaveolen poit as green corrosion inhibitor of corrosion of mild steel in 2 M HCl Journal of Emerging Trends in Engineering and Applied Sciences, 8(5), 201 – 205. |
| [42] | Adejo S O; Ekwenchi M M; Ahile J U; Gbertyo J A; Kaio. A., 2014, Proposing a new empirical adsorption isotherm known as Adejo-Ekwenchi isotherm, Journal of Applied Chemistry, 6(5), 66-71. |
| [43] | Li, Y.; Zhao, P.; Liang, Q.; Hou, B., 2005, Berberine as a natural source inhibitor for mild steel in 1 M H2SO4, Applied. Surface Science, 252(5), 1245-1253. |
| [44] | Gomma G.K., 1998, Mechanism of corrosion behaviour of carbon steel in tartaric and malic acid in the presence of Fe2+ ion, Materials Chemistry and Physics, 52, 200-206. |
| [45] | M. Lebrini, M. Lagrenée, H. Vezin, M. Traisnel, and F. Bentiss., 2007, Experimental and theoretical study for corrosion inhibition of mild steel in normal hydrochloric acid solution by some new macrocyclic polyether compounds. Corrosion Science, 49(5), 2254-2269. |
| [46] | Pavithra, M. K., Venkatesha, T. V., Kumar M. K. P., and Shivayogiraju. B. S., 2013, Acalypha torta Leaf Extract as Green Corrosion Inhibitor for Mild Steel in Hydrochloric Acid Solution. Industrial & Engineering Chemistry Research., 52(2), 722-728. |
| [47] | Koopmans T., 1934, Über T. Die Zuordnung von Wellenfunktionen und Eigenwerten zu den Einzelnen Elektronen Eines. Atoms. Physica; 1(1-6): 104–13. |
| [48] | Parr RG, Pearson RG. 1983, Absolute hardness: companion parameter to absolute electronegativity. Journal of the American Chemical society, 105(26): 7512-7516. |
| [49] | Parr RG, Szentpaly LL, Liu S., 1999, Electrophilicity index Journal of the American Chemical society, 121(9): 1922-1924. |
| [50] | Pearson RG., 1988, Absolute Electronegativity and Hardness: application to Inorganic Chemistry, Inorganic Chemistry, 27(4): 734-740. |
| [51] | M. J. S. Dewar, E. G. Zoebisch, E. F. Healy, J. P. Stewart, 1985, Development and use of quantum mechanical molecular models, 76, AM1: a new general purpose quantum mechanical molecular model, Journal of the American Chemical Society, 107, 3902-3909. |
| [52] | Kaya S., Kaya C., Guo L., Kandemirli F, Tüzün B., U ˘gurlu ˙I., et al., 2016, Quantum chemical and molecular dynamics simulation studies on inhibition performances of some thiazole and thiadiazole derivatives against corrosion of iron. Journal of Molecular Liquids; 219: 497–504. |
| [53] | Tigori, M., Kouyaté, A., Kouakou, V., Niamien, P., & Trokourey, A., 2020), Computational approach for predicting the adsorption properties and inhibition of some antiretroviral drugs on copper corrosion in HNO3. European Journal of Chemistry, 11(3), 235-244. |
| [54] | W. Yang, R.G. Parr, Hardness, softness, and the fukui function in the electronic theory of metals and catalysis. Proc. Natl. Acad. Sci. U.S.A., 1985, 82, 6723. |
| [55] | N. Mohanapriya, M. Kumaravel and B. Lalithamani, 2020, Theoretical and Experimental Studies on the Adsorption of N- [(E)-Pyridin-2-ylmethylidene] Aniline, a Schiff Base, on Mild Steel Surface in Acid Media. Journal of Electrochemical Science and Technology 11(2), 117-131. |
| [56] | M. Lagren´ee, B. Mernari, N. Chaibi, M. Traisnel, H. Vezin, and F. Bentiss, 2011, Investigation of the inhibitive effect of substituted oxadiazoles on the corrosion of mild steel in HCl medium, Corrosion Science, 43(5), 951–962. |
| [57] | M. A. Quraishi and R. Sardar, 2003, Hector bases — a new class of heterocyclic corrosion inhibitors for mild steel in acid solutions, Journal of Applied Electrochemistry, 33(12), 1163–1168. |
| [58] | K. F. Khaled, K. Babi´ c-Samardˇzija, and N. Hackerman, 2005, Theoretical study of the structural effects of polymethylene amines on corrosion inhibition of iron in acid solutions,” Electrochimica Acta, 50(12), 2515–2520. |
| [59] | Khaled, K.F., 2008, Molecular Simulation, Quantum Chemical Calculations and Electrochemical Studies for Inhibition of Mild Steel by Triazoles. Electrochimica Acta, 53(9), 3484-3492(2008). |
| [60] | B. Gomez, N.V. Likhanova, M.A. Dominguez-Aguilar, R. Martinez-Palou, A. Vela, J.L. 2006, Gazquez, Quantum Chemical Study of the Inhibitive Properties of 2-Pyridyl-Azoles, Journal of Physical Chemistry B, 110(18), 8928–8934. |
| [61] | G. Bereket, E. Hu¨r, and C. O¨ retir, “Quantum chemical studies on some imidazole derivatives as corrosion inhibitors for iron in acidic medium,” Journal of Molecular Structure: THEOCHEM, 578(1–3), 79–88. |
| [62] | Yang W, Mortier WJ, 1986 The use of global and local molecular parameters for the analysis of the gas-phase basicity of amines, Journal of the American Chemical Society, 108(19), 5708–5711. |
| [63] | Martínez-Araya J.I., 2015, Why the dual descriptor is a more accurate local reactivity descriptor than Fukui functions? journal of Mathematical Chemistry, 53: 451–465. |
| [64] | Christophe Morell André Grand Alejandro Toro-Labbé, 2004, New Dual Descriptor for Chemical Reactivity. Journal of Physical Chemistry. A, 109(1): 205–212. |


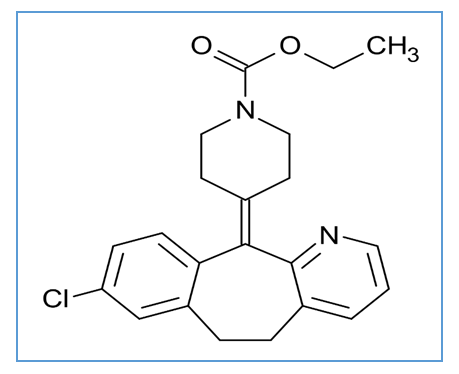
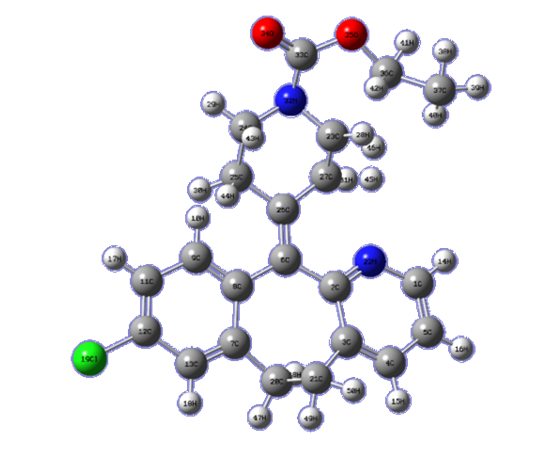
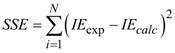
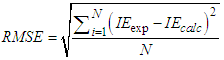
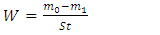
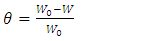
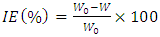
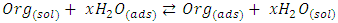
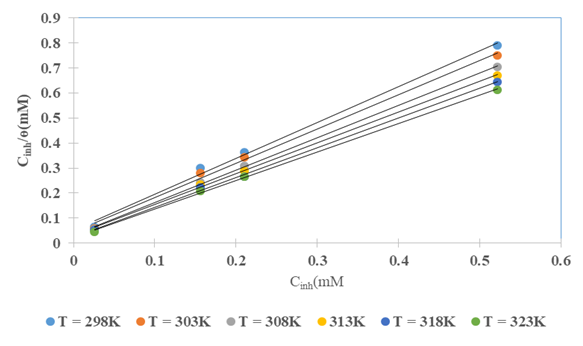
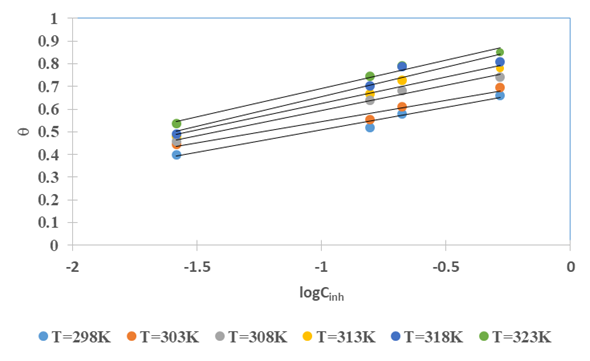
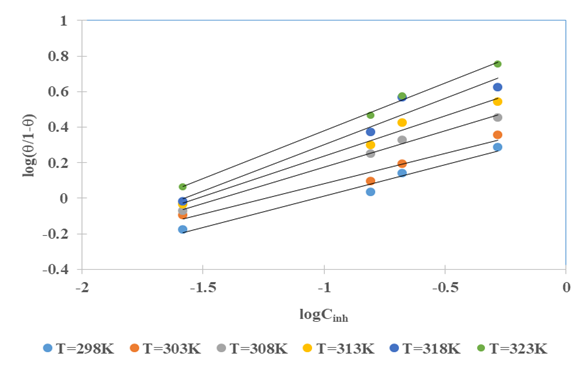
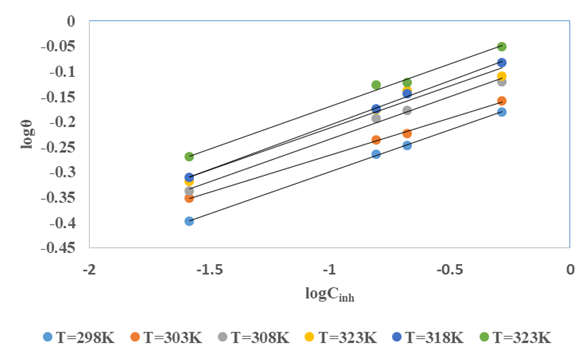
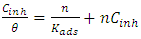
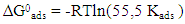
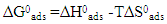
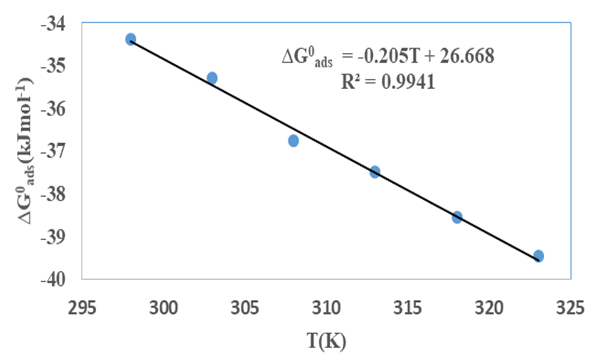
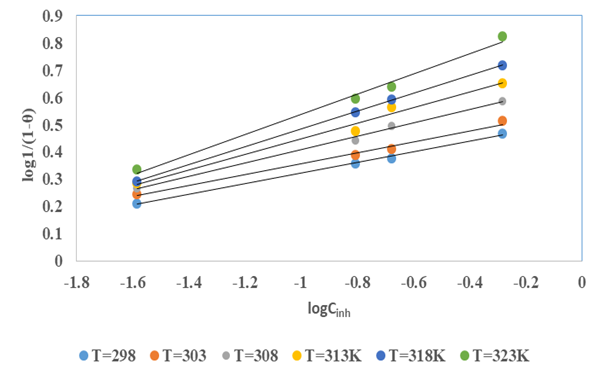

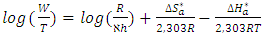
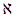 the Avogadro number The transition state plots of
the Avogadro number The transition state plots of 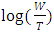 versus
versus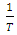 is given in Figure 12.
is given in Figure 12.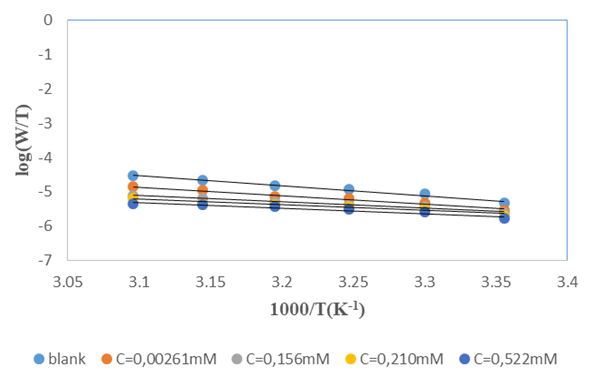
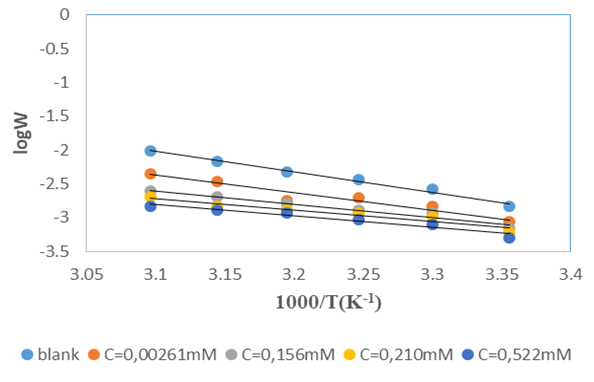
 and intercepts
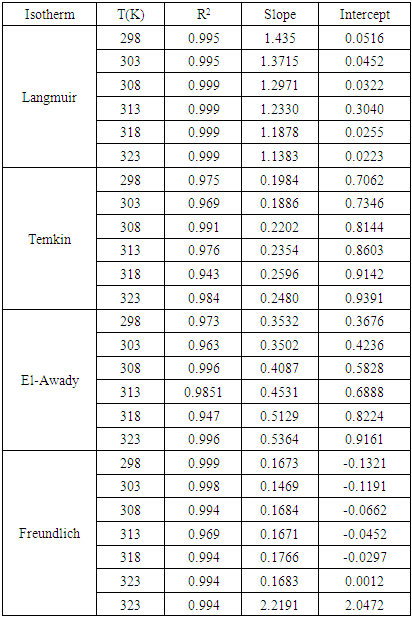
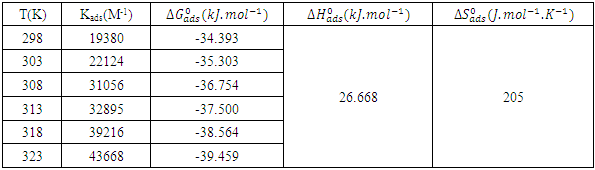
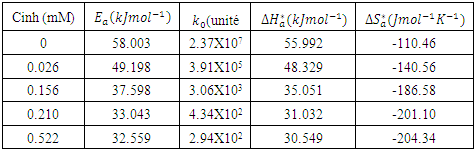
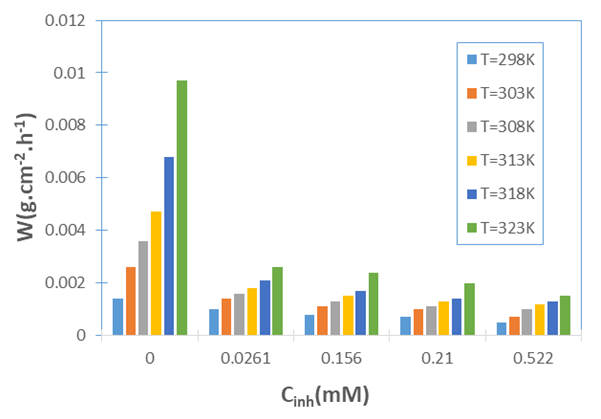
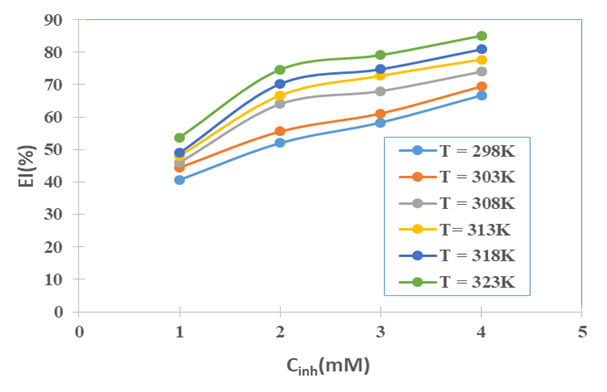
and intercepts 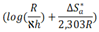 of the straight lines obtained. The obtained values are recorded in Table 5.The apparent activation energy (Ea) values in presence of loratadine are lower than the value obtained without this molecule (Cinh = 0). These observations reveal that the inhibition of corrosion reactions is influenced by chemisorption [44]. Ea decreases when the concentration of inhibitor increases, favouring the Cu2+ ions formation and thus the formation of the Cu-Inh complex leading to the reduction of corrosion phenomenon at high temperature. Indeed, the inhibitor adsorbs on metal surface by chemical bonds which are strong (chemisorption) and resists at high temperature. These observations justify the good performance of the molecule when the temperature increases. The values from ∆H*a are positive and are increasingly lower in presence of loratadine. This reflects an endothermic dissolution process leading to a slow dissolution of copper in the solution studied [45]. The negative sign of ∆S*a implies that the disorder decreases from reactant to activated complex [46].
of the straight lines obtained. The obtained values are recorded in Table 5.The apparent activation energy (Ea) values in presence of loratadine are lower than the value obtained without this molecule (Cinh = 0). These observations reveal that the inhibition of corrosion reactions is influenced by chemisorption [44]. Ea decreases when the concentration of inhibitor increases, favouring the Cu2+ ions formation and thus the formation of the Cu-Inh complex leading to the reduction of corrosion phenomenon at high temperature. Indeed, the inhibitor adsorbs on metal surface by chemical bonds which are strong (chemisorption) and resists at high temperature. These observations justify the good performance of the molecule when the temperature increases. The values from ∆H*a are positive and are increasingly lower in presence of loratadine. This reflects an endothermic dissolution process leading to a slow dissolution of copper in the solution studied [45]. The negative sign of ∆S*a implies that the disorder decreases from reactant to activated complex [46].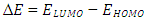


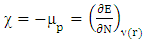
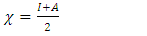
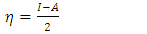
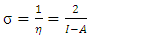
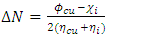
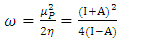
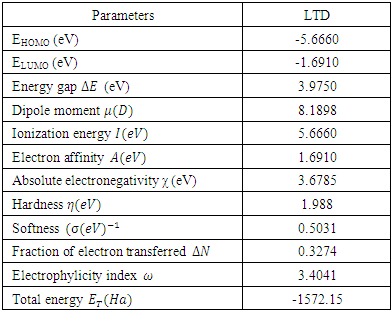
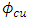 and
and 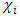 denote respectively the absolute electronegativity of copper and the inhibitor,
denote respectively the absolute electronegativity of copper and the inhibitor, 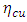 and
and 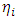 are respectively the global hardness of copper and the inhibitor. In this work Δ𝑁 has been determined using
are respectively the global hardness of copper and the inhibitor. In this work Δ𝑁 has been determined using  = 4.98 eV [50] and
= 4.98 eV [50] and  = 0 [51], assuming that for a metallic bulk
= 0 [51], assuming that for a metallic bulk 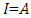 because they are softer than the neutral metallic atoms. The higher energy of HOMO (EHOMO) value, the greater is the tendency of the molecule to offer electrons to unoccupied orbital of the metal; in addition, the lower the energy of LUMO (ELUMO), the higher is the affinity for accepting electrons from the metal surface [52]. The inhibition potential of a reacting organic compound can be evaluated by the orbital energy difference. Furthermore, the smaller of orbital energy difference between the interacting orbitals, i.e. HOMO and LUMO (∆𝐸 = 𝐸𝐿𝑈𝑀𝑂 − 𝐸𝐻𝑂𝑀𝑂) would promote strong metal-molecule interaction [53]. In our case, the high value of EHOMO (-5.6660eV) and the low values of ELUMO (-1.6910eV) and ∆E (3.9750eV) of loratadine justify the high inhibition efficiency values obtained experimentally. Global hardness (η) and softness (σ) are related to inhibition efficiency which also depend on the energy gap. In fact a good inhibitor has a high softness value and a low hardness value [54,55]. Loratadine has a low hardness value (η = 1.988 eV) and a high softness value [σ = 0.5031 (eV)-1] is expected to have a high inhibition efficiency. These values are in agreement with the experimental results.The absolute electronegativity (χ) is the chemical property that describes the ability of a molecule to attract electron towards itself in a covalent bond [50]. The electronegativity value of loratadine is lower than copper, which confirms the electrons movement from loratadine to copper.For dipole moment
because they are softer than the neutral metallic atoms. The higher energy of HOMO (EHOMO) value, the greater is the tendency of the molecule to offer electrons to unoccupied orbital of the metal; in addition, the lower the energy of LUMO (ELUMO), the higher is the affinity for accepting electrons from the metal surface [52]. The inhibition potential of a reacting organic compound can be evaluated by the orbital energy difference. Furthermore, the smaller of orbital energy difference between the interacting orbitals, i.e. HOMO and LUMO (∆𝐸 = 𝐸𝐿𝑈𝑀𝑂 − 𝐸𝐻𝑂𝑀𝑂) would promote strong metal-molecule interaction [53]. In our case, the high value of EHOMO (-5.6660eV) and the low values of ELUMO (-1.6910eV) and ∆E (3.9750eV) of loratadine justify the high inhibition efficiency values obtained experimentally. Global hardness (η) and softness (σ) are related to inhibition efficiency which also depend on the energy gap. In fact a good inhibitor has a high softness value and a low hardness value [54,55]. Loratadine has a low hardness value (η = 1.988 eV) and a high softness value [σ = 0.5031 (eV)-1] is expected to have a high inhibition efficiency. These values are in agreement with the experimental results.The absolute electronegativity (χ) is the chemical property that describes the ability of a molecule to attract electron towards itself in a covalent bond [50]. The electronegativity value of loratadine is lower than copper, which confirms the electrons movement from loratadine to copper.For dipole moment 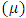 , several authors state that the inhibition efficiency increases with increasing values of this parameter [56,57]. Moreover, survey of the literature reveals that several irregularities appeared in case of correlation of dipole moment with inhibitor efficiency [58,59]. So in general, there is no significant relationship between the dipole moment values and inhibition efficiencies.The fraction of electrons transferred (ΔN) of a molecule reflects its ability to give electrons. According to Lukovits' study, if ΔN < 3.6 then the efficiency of inhibition increases with the molecule's ability to give electrons to the metal [59]. In our work ΔN < 3.6, which shows that loratadine has a good inhibition performance in electron donation.The electrophilicity index (ω) measures the propensity of chemical species to accept electrons. A high value of (ω) [49] describes a good electrophile, while a low value of (ω) describes a good nucleophile. In our case, the electrophilicity index of the molecule is high, expressing that loratadine is a good electrophile.Loratadine has negative value of total energy (ET < 0) and positive value of hardness (η > 0), which proves that the charge transfer from each molecule to the metal is energetically favorable [60]. So, there is a strong interaction between the molecules and the copper surface.Local reactivity was analyzed by means of Fukui indices and dual descriptor in order to assess the nucleophilic and electrophilic attack centre.The Fukui [61,62] functions expressed using the limit difference approximation is given as followsFor nucleophilic attack
, several authors state that the inhibition efficiency increases with increasing values of this parameter [56,57]. Moreover, survey of the literature reveals that several irregularities appeared in case of correlation of dipole moment with inhibitor efficiency [58,59]. So in general, there is no significant relationship between the dipole moment values and inhibition efficiencies.The fraction of electrons transferred (ΔN) of a molecule reflects its ability to give electrons. According to Lukovits' study, if ΔN < 3.6 then the efficiency of inhibition increases with the molecule's ability to give electrons to the metal [59]. In our work ΔN < 3.6, which shows that loratadine has a good inhibition performance in electron donation.The electrophilicity index (ω) measures the propensity of chemical species to accept electrons. A high value of (ω) [49] describes a good electrophile, while a low value of (ω) describes a good nucleophile. In our case, the electrophilicity index of the molecule is high, expressing that loratadine is a good electrophile.Loratadine has negative value of total energy (ET < 0) and positive value of hardness (η > 0), which proves that the charge transfer from each molecule to the metal is energetically favorable [60]. So, there is a strong interaction between the molecules and the copper surface.Local reactivity was analyzed by means of Fukui indices and dual descriptor in order to assess the nucleophilic and electrophilic attack centre.The Fukui [61,62] functions expressed using the limit difference approximation is given as followsFor nucleophilic attack 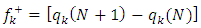

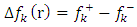
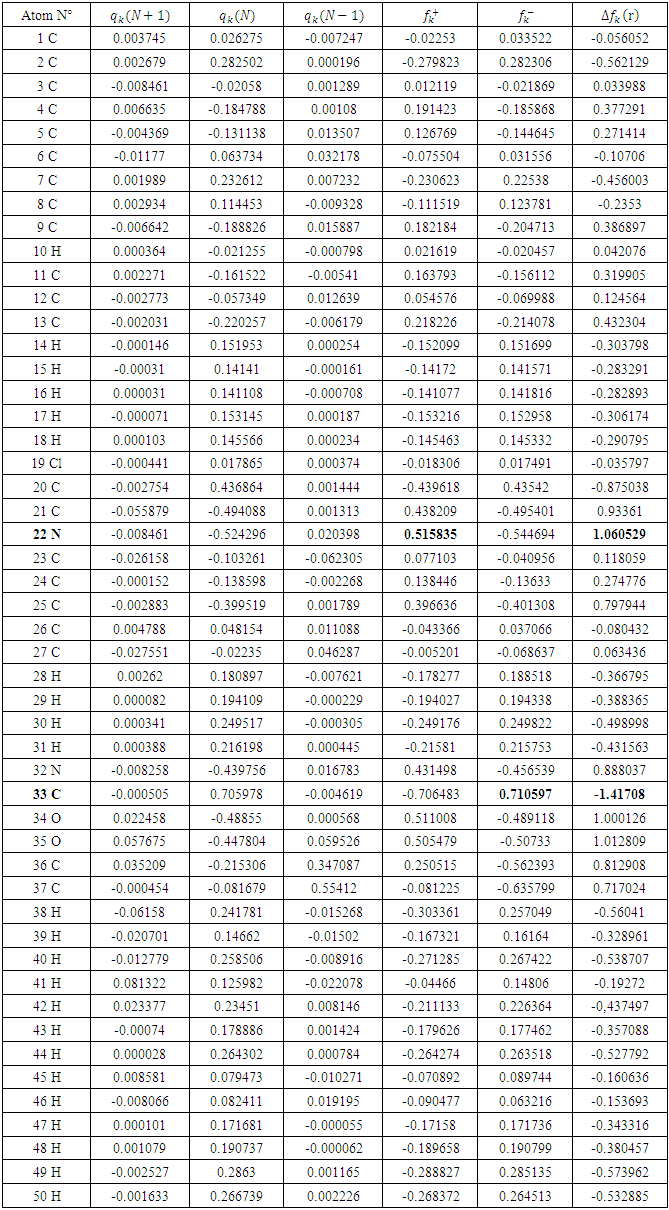
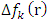 .All the local parameters are collected in Table 7.
.All the local parameters are collected in Table 7. and
and 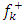 is the most probable nucleophilic attack site while C(33) with the maximum value of
is the most probable nucleophilic attack site while C(33) with the maximum value of 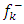 and the lowest value of
and the lowest value of 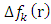 is most probable electrophilic attack site.The HOMO-LUMO diagrams are presenting by Figure 13.
is most probable electrophilic attack site.The HOMO-LUMO diagrams are presenting by Figure 13.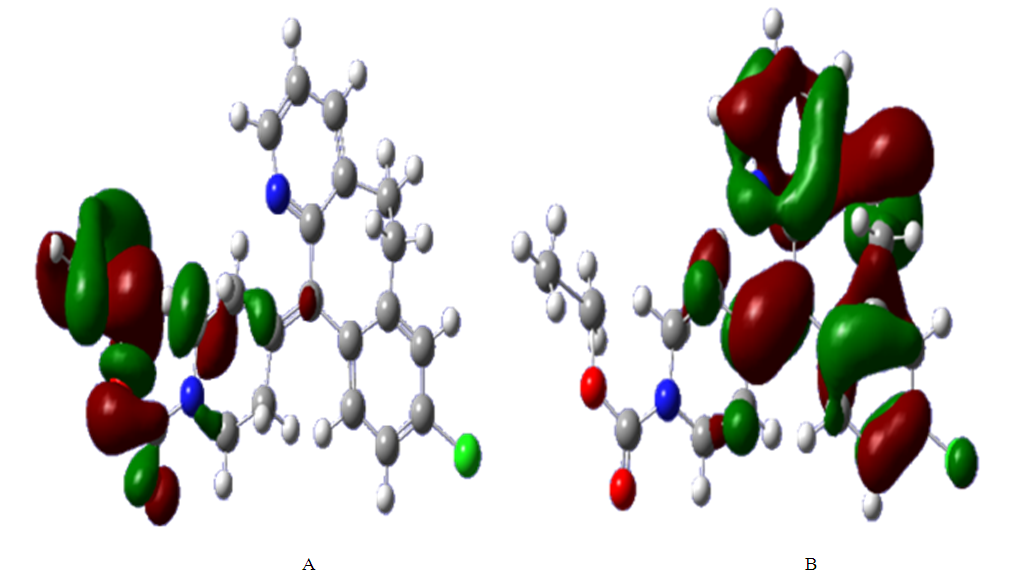
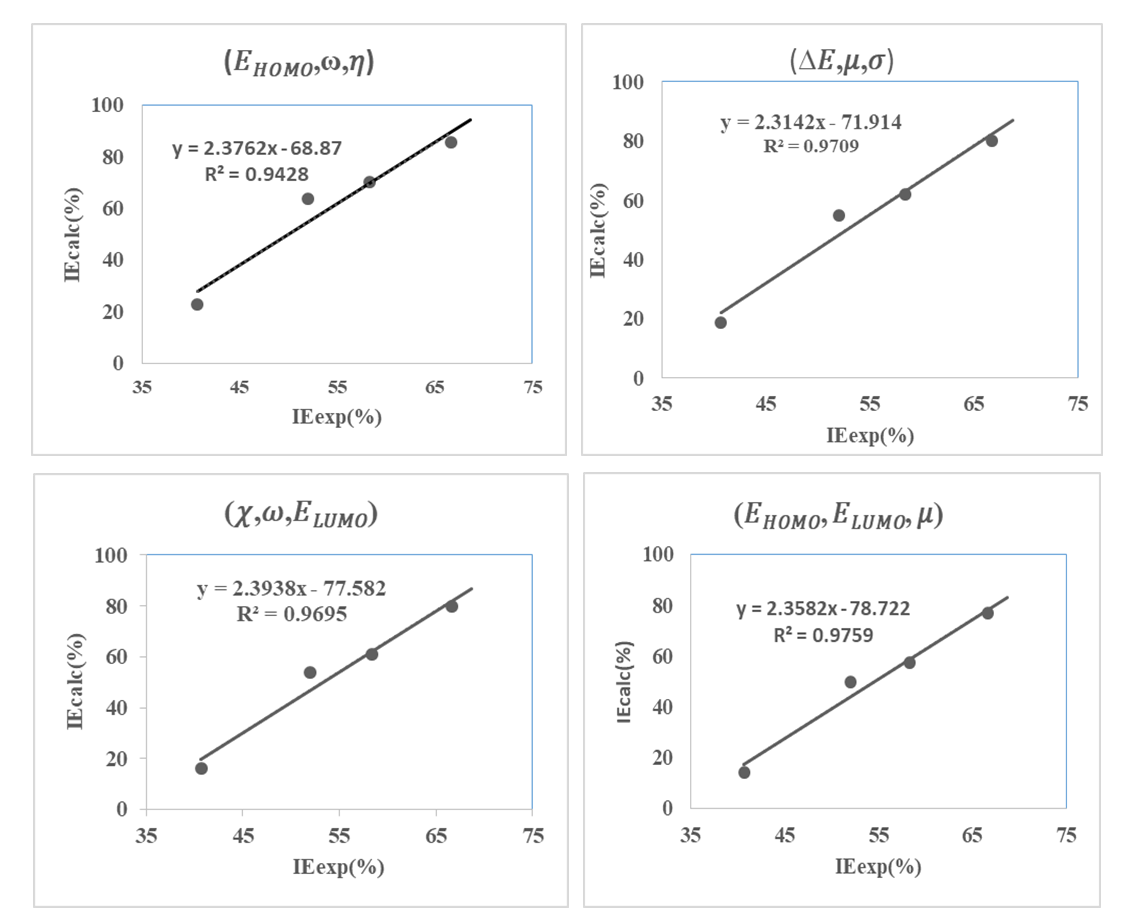
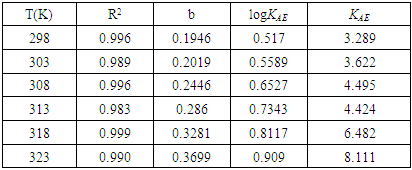
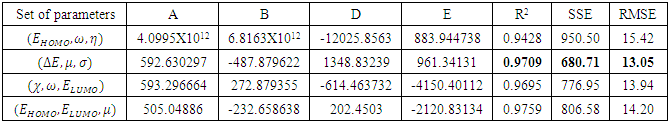
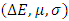 with the lowest value of RMSE and correlation coefficient R2=0.9709 is the best parameter to describe loratadine behavior of copper corrosion inhibition in 1M HNO3.
with the lowest value of RMSE and correlation coefficient R2=0.9709 is the best parameter to describe loratadine behavior of copper corrosion inhibition in 1M HNO3.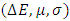 is the best set of parameters for correlating theoretical and experimental inhibitory efficiencies.• Calculated theoretical parameters support the experimental results.
is the best set of parameters for correlating theoretical and experimental inhibitory efficiencies.• Calculated theoretical parameters support the experimental results. Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML